Efficacy and Safety of Romosozumab Among Postmenopausal Women With Osteoporosis and Mild-to-Moderate Chronic Kidney Disease
- PMID: 35466448
- PMCID: PMC9544335
- DOI: 10.1002/jbmr.4563
Efficacy and Safety of Romosozumab Among Postmenopausal Women With Osteoporosis and Mild-to-Moderate Chronic Kidney Disease
Abstract
Patients with osteoporosis and chronic kidney disease (CKD) are at increased risk of fracture and associated negative outcomes, including increased mortality. The present post hoc analysis of two randomized, multicenter, phase 3 clinical trials-Fracture Study in Postmenopausal Women with Osteoporosis (FRAME) and Active-Controlled Fracture Study in Postmenopausal Women with Osteoporosis at High Risk (ARCH)-investigated the efficacy and safety of romosozumab in postmenopausal women with osteoporosis and mild-to-moderate CKD. The analysis included data from 7147 patients from FRAME and 4077 from ARCH. Eighty-one percent of patients from FRAME and 85% from ARCH had mild or moderate reduction in estimated glomerular filtration rate (eGFR) at baseline, and part of this reduction is likely age related. During the 1-year double-blind phases of the trials, patients received romosozumab 210 mg sc or placebo monthly in FRAME and romosozumab 210 mg sc monthly or alendronate 70 mg po weekly in ARCH. Bone mineral density (BMD) at the lumbar spine, total hip, and femoral neck and vertebral and nonvertebral fractures were assessed at baseline and month 12. In both trials, the least-square mean percent change from baseline BMD was significantly greater in the romosozumab groups versus controls across all kidney function categories at month 12. Romosozumab reduced the relative risk of new vertebral fractures at month 12 among patients with eGFR of 30-59, 60-89, and ≥90 mL/min by 72% (95% confidence interval [CI] 14-91; p = 0.017), 70% (40-85; p < 0.001), and 84% (30-96; p = 0.005), respectively, in FRAME versus placebo, and by 51% (5-75; p = 0.04), 19% (-28 to 49; p = 0.39), and 57% (1-81, p = 0.04), respectively, in ARCH versus alendronate. Incidences of adverse events, asymptomatic decreases in serum calcium, and evolution of kidney function during the studies were similar across all baseline kidney function groups. Romosozumab is an effective treatment option for postmenopausal women with osteoporosis and mild-to-moderate reduction in kidney function, with a similar safety profile across different levels of kidney function. © 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: ANABOLICS; CHRONIC KIDNEY DISEASE; MENOPAUSE; OSTEOPOROSIS; ROMOSOZUMAB.
© 2022 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Figures
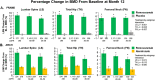

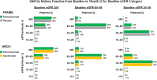
Comment in
-
Romosozumab and Renal Function.J Bone Miner Res. 2022 Aug;37(8):1435-1436. doi: 10.1002/jbmr.4645. Epub 2022 Jul 23. J Bone Miner Res. 2022. PMID: 35869697 No abstract available.
-
Romosozumab Use and Cardiovascular Events.J Bone Miner Res. 2023 Mar;38(3):452-453. doi: 10.1002/jbmr.4695. Epub 2022 Sep 20. J Bone Miner Res. 2023. PMID: 36127855 No abstract available.
-
Cardiovascular Safety of Romosozumab: New Insights from Postmenopausal Women with Chronic Kidney Disease.J Bone Miner Res. 2023 Feb;38(2):354-355. doi: 10.1002/jbmr.4724. Epub 2022 Nov 4. J Bone Miner Res. 2023. PMID: 36330828 No abstract available.
-
What is the risk of cardiovascular events in osteoporotic patients treated with romosozumab?Expert Opin Drug Saf. 2022 Dec;21(12):1441-1443. doi: 10.1080/14740338.2022.2160445. Epub 2022 Dec 27. Expert Opin Drug Saf. 2022. PMID: 36538034 No abstract available.
-
Reply to Letter to the Editor: Efficacy and Safety of Romosozumab Among Postmenopausal Women With Osteoporosis and Mild-to-Moderate Chronic Kidney Disease.J Bone Miner Res. 2023 Feb;38(2):356. doi: 10.1002/jbmr.4761. Epub 2023 Jan 25. J Bone Miner Res. 2023. PMID: 36698050 No abstract available.
References
-
- National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases . Kidney disease statistics for the United States. Available at: https://www.niddk.nih.gov/health-information/health-statistics/kidney-di.... Accessed April 1, 2020.
-
- National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases . 2019. USRDS annual data report: epidemiology of kidney disease in the United States. Available at: https://www.usrds.org/media/2371/2019-executive-summary.pdf. Accessed April 1, 2020.
-
- Klawansky S, Komaroff E, Cavanaugh PF Jr, Mitchell DY, Gordon MJ. Relationship between age, renal function and bone mineral density in the US population. Osteoporos Int. 2003;14(7):570‐573. - PubMed
-
- Bover J, Bailone L, Lopez‐Baez V, et al. Osteoporosis, bone mineral density and CKD‐MBD: treatment considerations. J Nephrol. 2017;30(5):677‐687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

