Regulating Dendrite-Free Zinc Deposition by Red Phosphorous-Derived Artificial Protective Layer for Zinc Metal Batteries
- PMID: 35466570
- PMCID: PMC9218763
- DOI: 10.1002/advs.202200155
Regulating Dendrite-Free Zinc Deposition by Red Phosphorous-Derived Artificial Protective Layer for Zinc Metal Batteries
Abstract
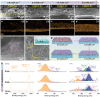
Rational architecture design of the artificial protective layer on the zinc (Zn) anode surface is a promising strategy to achieve uniform Zn deposition and inhibit the uncontrolled growth of Zn dendrites. Herein, a red phosphorous-derived artificial protective layer combined with a conductive N-doped carbon framework is designed to achieve dendrite-free Zn deposition. The Zn-phosphorus (ZnP) solid solution alloy artificial protective layer is formed during Zn plating. Meanwhile, the dynamic evolution mechanism of the ZnP on the Zn anode is successfully revealed. The concentration gradient of the electrolyte on the electrode surface can be redistributed by this protective layer, thereby achieving a uniform Zn-ion flux. The fabricated Zn symmetrical battery delivers a dendrite-free plating/stripping for 1100 h at the current density of 2.0 mA cm-2 . Furthermore, aqueous Zn//MnO2 full cell exhibits a reversible capacity of 200 mAh g-1 after 350 cycles at 1.0 A g-1 . This study suggests an effective solution for the suppression of Zn dendrites in Zn metal batteries, which is expected to provide a deep insight into the design of high-performance rechargeable aqueous Zn-ion batteries.
Keywords: Zn anode; aqueous Zn-ion batteries; artificial protective layer; dendrite-free Zn deposition.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- a) Parker J. F., Chervin C. N., Pala I. R., Machler M., Burz M. F., Long J. W., Rolison D. R., Science 2017, 356, 415; - PubMed
- b) Jia X., Liu C., Neale Z. G., Yang J., Cao G., Chem. Rev. 2020, 120, 7795; - PubMed
- c) Li S., Fu J., Miao G., Wang S., Zhao W., Wu Z., Zhang Y., Yang X., Adv. Mater. 2021, 33, 2008424; - PubMed
- d) Kundu D., Adams B. D., Duffort V., Vajargah S. H., Nazar L. F., Nat. Energy 2016, 1, 16119;
- e) Li C., Sun Z., Yang T., Yu L., Wei N., Tian Z., Cai J., Lv J., Shao Y., Rummeli M. H., Sun J., Liu Z., Adv. Mater. 2020, 32, 2003425. - PubMed
-
- a) Yan H., Li S., Nan Y., Yang S., Li B., Adv. Energy Mater. 2021, 11, 2100186;
- b) Hao J., Li B., Li X., Zeng X., Zhang S., Yang F., Liu S., Li D., Wu C., Guo Z., Adv. Mater. 2020, 32, 2003021. - PubMed
-
- a) Xie F., Li H., Wang X., Zhi X., Chao D., Davey K., Qiao S. Z., Adv. Energy Mater. 2021, 11, 2003419;
- b) Zou P., Zhang R., Yao L., Qin J., Kisslinger K., Zhuang H., Xin H. L., Adv. Energy Mater. 2021, 11, 2100982;
- c) Zhang N., Chen X., Yu M., Niu Z., Cheng F., Chen J., Chem. Soc. Rev. 2020, 49, 4203. - PubMed
-
- a) Qi Zhang J. L., Tang Y., Ji X., Wang ., Angew. Chem., Int. Ed. 2020, 59, 13180; - PubMed
- b) Zhang X., Li J., Liu D., Liu M., Zhou T., Qi K., Shi L., Zhu Y., Qian Y., Energy Environ. Sci. 2021, 14, 3120;
- c) Yang Y., Liu C., Lv Z., Yang H., Zhang Y., Ye M., Chen L., Zhao J., Li C. C., Adv. Mater. 2021, 33, 2007388; - PubMed
- d) Zhang Q., Luan J., Huang X., Wang Q., Sun D., Tang Y., Ji X., Wang H., Nat. Commun. 2020, 11, 3961. - PMC - PubMed
-
- a) Xie X., Liang S., Gao J., Guo S., Guo J., Wang C., Xu G., Wu X., Chen G., Zhou J., Energy Environ. Sci. 2020, 13, 503;
- b) Hao J., Li X., Zeng X., Li D., Mao J., Guo Z., Energy Environ. Sci. 2020, 13, 3917;
- c) Qiu X., Wang N., Wang Z., Wang F., Wang Y., Angew. Chem., Int. Ed. 2021, 60, 2. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
