In-ovo imaging using ostrich eggs: Biomagnetism for detection of cardiac signals and embryonal motion
- PMID: 35466741
- PMCID: PMC9265528
- DOI: 10.1177/15353702221082046
In-ovo imaging using ostrich eggs: Biomagnetism for detection of cardiac signals and embryonal motion
Abstract
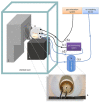
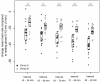
In-ovo imaging using ostrich eggs has been described as a potential alternative to common animal testing. The main advantage is its independence from small animal imaging devices as ostrich eggs provide good image quality on regular CT, MRI, or PET used in examinations of humans. However, embryonal motion during dynamic imaging studies produce artifacts. The aims of this study were (1) to explore the feasibility of biomagnetism to detect cardiac signals and embryonal motion and to use these findings (2) to investigate the effect of isoflurane anesthesia on ostrich embryos. A standard magnetoencephalography developed for brain studies was used to detect embryonal signals of ostrich eggs on developmental day 34. Signals were instantly shown on a screen and data were also postprocessed. For assessing the effects of anesthesia, nine ostrich eggs were investigated using isoflurane 6% for 90 min. Biomagnetic signals were recorded simultaneously. A control group consisting of eight different ostrich eggs was also investigated. Cardiac signals similar to electrocardiography were observed in all eggs. Postprocessing revealed frequent motion of embryos without anesthesia. The exposure to isoflurane led to a significant decrease in motion signals in 9/9 ostrich embryos after 8 min. Motion was significantly reduced in the isoflurane group versus control group. There were no isoflurane-related deaths. This study shows that biomagnetism is feasible to detect cardiac signals and motion of ostrich embryos in-ovo. Application of isoflurane is safe and leads to a rapid decrease in embryonal motion, which is an important prerequisite for the implementation of in-ovo imaging using ostrich eggs.
Keywords: In-ovo imaging; alternative animal testing; biomagnetism; isoflurane anesthesia; ostrich eggs.
Conflict of interest statement
Figures






References
-
- Freesmeyer M, Kuehnel C, Opfermann T, Niksch T, Wiegand S, Stolz R, Huonker R, Witte OW, Winkens T. The use of ostrich eggs for in ovo research: making preclinical imaging research affordable and available. J Nucl Med 2018;59:1901–6 - PubMed
-
- Winkens T, Christl A, Kuehnel C, Ndum F, Freesmeyer M. In-ovo imaging using ostrich eggs—evaluation of physiological embryonal development on computed tomography. Acta Zool 2021;0:1–11
-
- United States Department of Agriculture. Animal welfare act and animal welfare regulations, 2013, https://www.aphis.usda.gov/animal_welfare/downloads/bluebook-ac-awa.pdf
-
- Europäisches Parlament und Rat. Richtlinie 2010/63/EU zum Schutz der für wissenschaftliche Zwecke verwendeten Tiere, 2010, https://eur-lex.europa.eu/legal-content/DE/TXT/HTML/?uri=LEGISSUM:sa0027
-
- Bundesministerium der Justiz und für Verbraucherschutz. Tierschutzgesetz, 2016, https://www.gesetze-im-internet.de/tierschg/BJNR012770972.html
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

