Temperate Conditions Limit Zika Virus Genome Replication
- PMID: 35467365
- PMCID: PMC9131854
- DOI: 10.1128/jvi.00165-22
Temperate Conditions Limit Zika Virus Genome Replication
Abstract
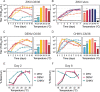
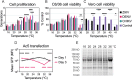
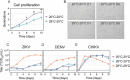
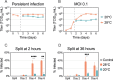
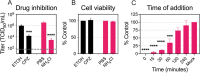
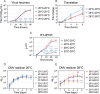
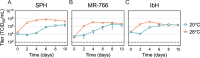
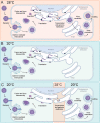
Zika virus is a mosquito-borne flavivirus known to cause severe birth defects and neuroimmunological disorders. We have previously demonstrated that mosquito transmission of Zika virus decreases with temperature. While transmission was optimized at 29°C, it was limited at cool temperatures (<22°C) due to poor virus establishment in the mosquitoes. Temperature is one of the strongest drivers of vector-borne disease transmission due to its profound effect on ectothermic mosquito vectors, viruses, and their interaction. Although there is substantial evidence of temperature effects on arbovirus replication and dissemination inside mosquitoes, little is known about whether temperature affects virus replication directly or indirectly through mosquito physiology. In order to determine the mechanisms behind temperature-induced changes in Zika virus transmission potential, we investigated different steps of the virus replication cycle in mosquito cells (C6/36) at optimal (28°C) and cool (20°C) temperatures. We found that the cool temperature did not alter Zika virus entry or translation, but it affected genome replication and reduced the amount of double-stranded RNA replication intermediates. If replication complexes were first formed at 28°C and the cells were subsequently shifted to 20°C, the late steps in the virus replication cycle were efficiently completed. These data suggest that cool temperature decreases the efficiency of Zika virus genome replication in mosquito cells. This phenotype was observed in the Asian lineage of Zika virus, while the African lineage Zika virus was less restricted at 20°C. IMPORTANCE With half of the human population at risk, arboviral diseases represent a substantial global health burden. Zika virus, previously known to cause sporadic infections in humans, emerged in the Americas in 2015 and quickly spread worldwide. There was an urgent need to better understand the disease pathogenesis and develop therapeutics and vaccines, as well as to understand, predict, and control virus transmission. In order to efficiently predict the seasonality and geography for Zika virus transmission, we need a deeper understanding of the host-pathogen interactions and how they can be altered by environmental factors such as temperature. Identifying the step in the virus replication cycle that is inhibited under cool conditions can have implications in modeling the temperature suitability for arbovirus transmission as global environmental patterns change. Understanding the link between pathogen replication and environmental conditions can potentially be exploited to develop new vector control strategies in the future.
Keywords: RNA replication; Zika virus; temperature.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Variable Inhibition of Zika Virus Replication by Different Wolbachia Strains in Mosquito Cell Cultures.J Virol. 2017 Jun 26;91(14):e00339-17. doi: 10.1128/JVI.00339-17. Print 2017 Jul 15. J Virol. 2017. PMID: 28446677 Free PMC article.
-
Structural and functional significance of Aedes aegypti AgBR1 flavivirus immunomodulator.J Virol. 2025 May 20;99(5):e0187824. doi: 10.1128/jvi.01878-24. Epub 2025 Apr 24. J Virol. 2025. PMID: 40272158 Free PMC article.
-
Wolbachia wStri Blocks Zika Virus Growth at Two Independent Stages of Viral Replication.mBio. 2018 May 22;9(3):e00738-18. doi: 10.1128/mBio.00738-18. mBio. 2018. PMID: 29789369 Free PMC article.
-
Zika Virus Mosquito Vectors: Competence, Biology, and Vector Control.J Infect Dis. 2017 Dec 16;216(suppl_10):S976-S990. doi: 10.1093/infdis/jix405. J Infect Dis. 2017. PMID: 29267910 Free PMC article. Review.
-
Antiviral Compounds for Blocking Arboviral Transmission in Mosquitoes.Viruses. 2021 Jan 14;13(1):108. doi: 10.3390/v13010108. Viruses. 2021. PMID: 33466915 Free PMC article. Review.
Cited by
-
Distinct chikungunya virus polymerase palm subdomains contribute to viral protein accumulation and virion production.PLoS Pathog. 2024 Oct 14;20(10):e1011972. doi: 10.1371/journal.ppat.1011972. eCollection 2024 Oct. PLoS Pathog. 2024. PMID: 39401243 Free PMC article.
-
Decoding the Role of Temperature in RNA Virus Infections.mBio. 2022 Oct 26;13(5):e0202122. doi: 10.1128/mbio.02021-22. Epub 2022 Aug 18. mBio. 2022. PMID: 35980031 Free PMC article. Review.
-
Ellagic Acid as a Potential Inhibitor against the Nonstructural Protein NS3 Helicase of Zika Virus: A Molecular Modelling Study.Biomed Res Int. 2022 Aug 21;2022:2044577. doi: 10.1155/2022/2044577. eCollection 2022. Biomed Res Int. 2022. PMID: 36046457 Free PMC article.
-
Deep Mutational Scanning in Disease-related Genes with Saturation Mutagenesis-Reinforced Functional Assays (SMuRF).bioRxiv [Preprint]. 2024 Jun 25:2023.07.12.548370. doi: 10.1101/2023.07.12.548370. bioRxiv. 2024. Update in: Cell. 2024 Nov 14;187(23):6707-6724.e22. doi: 10.1016/j.cell.2024.08.047. PMID: 37873263 Free PMC article. Updated. Preprint.
-
Saturation mutagenesis-reinforced functional assays for disease-related genes.Cell. 2024 Nov 14;187(23):6707-6724.e22. doi: 10.1016/j.cell.2024.08.047. Epub 2024 Sep 25. Cell. 2024. PMID: 39326416
References
-
- Cao-Lormeau V-M, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, Vial A-L, Decam C, Choumet V, Halstead SK, Willison HJ, Musset L, Manuguerra J-C, Despres P, Fournier E, Mallet H-P, Musso D, Fontanet A, Neil J, Ghawché F. 2016. Guillain-Barre syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 387:1531–1539. 10.1016/S0140-6736(16)00562-6. - DOI - PMC - PubMed
-
- World Health Organization. 2016. WHO statement on the first meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations. http://www.who.int/mediacentre/news/statements/2016/1st-emergency-commit.... Accessed July 19, 2017.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

