Streptococcus pyogenes Hijacks Host Glutathione for Growth and Innate Immune Evasion
- PMID: 35467425
- PMCID: PMC9239160
- DOI: 10.1128/mbio.00676-22
Streptococcus pyogenes Hijacks Host Glutathione for Growth and Innate Immune Evasion
Abstract
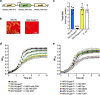
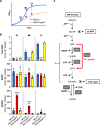
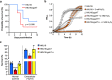
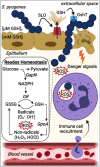
The nasopharynx and the skin are the major oxygen-rich anatomical sites for colonization by the human pathogen Streptococcus pyogenes (group A Streptococcus [GAS]). To establish infection, GAS must survive oxidative stress generated during aerobic metabolism and the release of reactive oxygen species (ROS) by host innate immune cells. Glutathione is the major host antioxidant molecule, while GAS is glutathione auxotrophic. Here, we report the molecular characterization of the ABC transporter substrate binding protein GshT in the GAS glutathione salvage pathway. We demonstrate that glutathione uptake is critical for aerobic growth of GAS and that impaired import of glutathione induces oxidative stress that triggers enhanced production of the reducing equivalent NADPH. Our results highlight the interrelationship between glutathione assimilation, carbohydrate metabolism, virulence factor production, and innate immune evasion. Together, these findings suggest an adaptive strategy employed by extracellular bacterial pathogens to exploit host glutathione stores for their own benefit. IMPORTANCE During infection, microbes must escape host immune responses and survive exposure to reactive oxygen species produced by immune cells. Here, we identify the ABC transporter substrate binding protein GshT as a key component of the glutathione salvage pathway in glutathione-auxotrophic GAS. Host-acquired glutathione is crucial to the GAS antioxidant defense system, facilitating escape from the host innate immune response. This study demonstrates a direct link between glutathione assimilation, aerobic metabolism, and virulence factor production in an important human pathogen. Our findings provide mechanistic insight into host adaptation that enables extracellular bacterial pathogens such as GAS to exploit the abundance of glutathione in the host cytosol for their own benefit.
Keywords: Streptococcus pyogenes; glutathione uptake; immune evasion; oxidative stress; redox homeostasis; virulence regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
T4 Pili Promote Colonization and Immune Evasion Phenotypes of Nonencapsulated M4 Streptococcus pyogenes.mBio. 2020 Jul 21;11(4):e01580-20. doi: 10.1128/mBio.01580-20. mBio. 2020. PMID: 32694142 Free PMC article.
-
The Transcriptional Regulator CpsY Is Important for Innate Immune Evasion in Streptococcus pyogenes.Infect Immun. 2017 Feb 23;85(3):e00925-16. doi: 10.1128/IAI.00925-16. Print 2017 Mar. Infect Immun. 2017. PMID: 27993974 Free PMC article.
-
Methods to Analyze the Contribution of Complement Evasion Factor (CEF) to Streptococcus pyogenes Virulence.Methods Mol Biol. 2023;2674:119-129. doi: 10.1007/978-1-0716-3243-7_8. Methods Mol Biol. 2023. PMID: 37258964
-
Mechanisms of group A Streptococcus resistance to reactive oxygen species.FEMS Microbiol Rev. 2015 Jul;39(4):488-508. doi: 10.1093/femsre/fuu009. Epub 2015 Feb 10. FEMS Microbiol Rev. 2015. PMID: 25670736 Free PMC article. Review.
-
Catch Me if You Can: Streptococcus pyogenes Complement Evasion Strategies.J Innate Immun. 2019;11(1):3-12. doi: 10.1159/000492944. Epub 2018 Sep 28. J Innate Immun. 2019. PMID: 30269134 Free PMC article. Review.
Cited by
-
Hidden in plain sight: How helminths manage to thrive in host blood.Front Parasitol. 2023 Mar 10;2:1128299. doi: 10.3389/fpara.2023.1128299. eCollection 2023. Front Parasitol. 2023. PMID: 39816845 Free PMC article. Review.
-
Diverse roles of low-molecular weight thiol GSH in Francisella's virulence, location sensing and GSH-stealing from host.Curr Res Microb Sci. 2023 Dec 20;6:100218. doi: 10.1016/j.crmicr.2023.100218. eCollection 2024. Curr Res Microb Sci. 2023. PMID: 38303966 Free PMC article.
-
Bacterial metabolism in the host and its association with virulence.Virulence. 2025 Dec;16(1):2459336. doi: 10.1080/21505594.2025.2459336. Epub 2025 Jan 31. Virulence. 2025. PMID: 39890585 Free PMC article. Review.
-
Group A Streptococcus interactions with the host across time and space.Curr Opin Microbiol. 2024 Feb;77:102420. doi: 10.1016/j.mib.2023.102420. Epub 2024 Jan 14. Curr Opin Microbiol. 2024. PMID: 38219421 Free PMC article. Review.
-
Resisting death by metal: metabolism and Cu/Zn homeostasis in bacteria.Emerg Top Life Sci. 2024 Feb 22;8(1):45-56. doi: 10.1042/ETLS20230115. Emerg Top Life Sci. 2024. PMID: 38362914 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
