Current State of Pediatric Reference Intervals and the Importance of Correctly Describing the Biochemistry of Child Development: A Review
- PMID: 35467725
- PMCID: PMC10155856
- DOI: 10.1001/jamapediatrics.2022.0794
Current State of Pediatric Reference Intervals and the Importance of Correctly Describing the Biochemistry of Child Development: A Review
Abstract
Importance: Appropriately established pediatric reference intervals are critical to the clinical decision-making process and should reflect the physiologic changes that occur during healthy child development. Reference intervals used in pediatric care today remain highly inconsistent across a broad range of common clinical biomarkers.
Observations: This narrative review assesses biomarker-specific pediatric reference intervals and their clinical utility with respect to the underlying biological changes occurring during development. Pediatric reference intervals from PubMed-indexed articles published from January 2015 to April 2021, commercial laboratory websites, study cohorts, and pediatric reference interval books were all examined. Although large numbers of pediatric reference intervals are published for some biomarkers, very few are used by clinical and commercial laboratories. The patterns, extent, and timing of biomarker changes are highly variable, particularly during developmental stages with rapid physiologic changes. However, many pediatric reference intervals do not capture these changes and thus do not accurately reflect the underlying biochemistry of development, resulting in significant inconsistencies between reference intervals.
Conclusions and relevance: There is a need to correctly describe the biochemistry of child development as well as to identify strategies to develop accurate and consistent pediatric reference intervals for improved pediatric care.
Conflict of interest statement
Figures
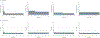
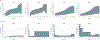
Comment in
-
Defining Normal.JAMA Pediatr. 2022 Jul 1;176(7):644-645. doi: 10.1001/jamapediatrics.2022.0801. JAMA Pediatr. 2022. PMID: 35467711 No abstract available.
References
-
- Wong E, Brungnara C, Straseski J, Kellogg M, Khosrow A. Pediatric Reference Intervals. 8th ed: Academic Press; 2020.
-
- Léger J, Olivieri A, Donaldson M, et al.; ESPE-PES-SLEP-JSPE-APEG-APPES-ISPAE; Congenital Hypothyroidism Consensus Conference Group. European Society for Paediatric Endocrinology consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism. J Clin Endocrinol Metab. 2014;99 (2):363–384. doi:10.1210/jc.2013-1891 - DOI - PMC - PubMed
-
- Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory: CLSI document EP28-A3c. Clinical and Laboratory Standards Institute; 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

