Selumetinib in children with neurofibromatosis type 1 and asymptomatic inoperable plexiform neurofibroma at risk for developing tumor-related morbidity
- PMID: 35467749
- PMCID: PMC9629448
- DOI: 10.1093/neuonc/noac109
Selumetinib in children with neurofibromatosis type 1 and asymptomatic inoperable plexiform neurofibroma at risk for developing tumor-related morbidity
Abstract
Background: Selumetinib was recently approved for the treatment of inoperable symptomatic plexiform neurofibromas (PNs) in children with neurofibromatosis type 1 (NF1). This parallel phase II study determined the response rate to selumetinib in children with NF1 PN without clinically significant morbidity.
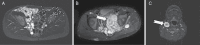
Methods: Children with NF1 and inoperable PNs, which were not yet causing clinically significant morbidity but had the potential to cause symptoms, received selumetinib at 25 mg/m2 orally twice daily (1 cycle = 28 days). Volumetric magnetic resonance imaging analysis and outcome assessments, including patient-reported (PRO), observer-reported, and functional outcome measures were performed every 4 cycles for 2 years, with changes assessed over time. A confirmed partial response (cPR) was defined as PN volume decrease of ≥20% on at least 2 consecutive scans ≥3 months apart.
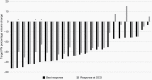
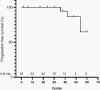
Results: 72% of subjects experienced a cPR on selumetinib. Participants received selumetinib for a median of 41 cycles (min 2, max 67) at data cutoff. Approximately half of the children rated having some target tumor pain at baseline, which significantly decreased by pre-cycle 13. Most objectively measured baseline functions, including visual, motor, bowel/bladder, or airway function were within normal limits and did not clinically or statistically worsen during treatment.
Conclusions: Selumetinib resulted in PN shrinkage in most subjects with NF1 PN without clinically significant morbidity. No new PN-related symptoms developed while on selumetinib, and PRO measures indicated declines in tumor-related pain intensity. This supports that selumetinib treatment may prevent the development of PN-related morbidities, though future prospective studies are needed to confirm these results.
Clinical trial registration: ClinicalTrials.gov NCT01362803.
Keywords: MEK inhibitor; neurofibromatosis type 1; patient-reported outcome measures; plexiform neurofibroma; selumetinib.
Published by Oxford University Press on behalf of the Society for Neuro-Oncology 2022.
Figures
References
-
- Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. Neurofibromatosis type 1 revisited. Pediatrics. 2009;123(1):124–133. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

