Maternal beta-blocker dose and risk of small-for gestational-age in women with heart disease
- PMID: 35467752
- PMCID: PMC9564806
- DOI: 10.1111/aogs.14363
Maternal beta-blocker dose and risk of small-for gestational-age in women with heart disease
Abstract
Introduction: Beta-blockers are prescribed for many pregnant women with heart disease, but whether there is a dose-dependent effect on fetal growth remains to be examined. We aimed to investigate if antenatal beta-blocker use and dose were associated with delivering a small-for-gestational-age infant among women with heart disease.
Material and methods: Our cohort included women with heart disease who delivered at Oslo University Hospital between 2006 and 2015. Maternal heart disease was classified into modified WHO risk scores. Women with beta-blocker treatment were dichotomized into whether they had been treated with a low or high dose based on clinical factors. We compared the risk of delivering a small-for-gestational-age infant in women exposed to high doses, low doses, or with no exposure to antenatal beta-blockers while adjusting for severity of maternal heart disease in logistic regression models.
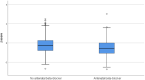
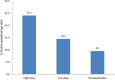
Results: Of a total of 540 pregnancies among women with heart disease, 163 (30.2%) were exposed to beta-blocker treatment. The majority were treated with metoprolol (86.5%). Almost twice as many babies in the beta-blocker group were small-for-gestational-age, compared with the non-exposed group (19.8 vs 9.5%, P < 0.001). Women using a high-dose beta-blocker had a five-fold increased risk of delivering a small-for-gestational-age infant compared with non-exposure (adjusted odds ratio [aOR] 4.89, 95% confidence interval [CI] 2.22-10.78, P < 0.001). Women using a low dose of beta-blocker had a two-fold increased risk of delivering a small-for-gestational-age infant; however, the confidence interval included the null (aOR 1.75, 95% CI 0.83-3.72, P = 0.143). Results when restricting the analyses to metoprolol showed the same pattern, but with attenuation of risks.
Conclusions: We found a five-fold increased risk of delivering a small-for-gestational-age infant in women with heart disease treated with a high dose of beta-blocker, and a two-fold increased risk among those treated with a low dose, showing an apparent dose-response relation. Close monitoring of fetal growth is warranted among women with heart disease treated with beta-blockers. As drug therapy in pregnancy concerns both mother and fetus, an optimum balance for both should be the goal.
Keywords: beta-blocker; birthweight; heart disease; modified World Health Organization risk score; pregnancy; small-for-gestational-age; z score.
© 2022 The Authors. Acta Obstetricia et Gynecologica Scandinavica published by John Wiley & Sons Ltd on behalf of Nordic Federation of Societies of Obstetrics and Gynecology (NFOG).
Conflict of interest statement
The authors report no conflicts of interest.
Figures
References
-
- Nyflot LT, Johansen M, Mulic‐Lutvica A, et al. The impact of cardiovascular diseases on maternal deaths in the Nordic countries. Acta Obstet Gynecol Scand. 2021;100:1273‐1279. - PubMed
-
- Schutte JM, Steegers EA, Schuitemaker NW, et al. Rise in maternal mortality in The Netherlands. BJOG. 2010;117:399‐406. - PubMed
-
- Regitz‐Zagrosek V, Roos‐Hesselink JW, Bauersachs J, et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165‐3241. - PubMed
-
- Bottega N, Malhame I, Guo L, Ionescu‐Ittu R, Therrien J, Marelli A. Secular trends in pregnancy rates, delivery outcomes, and related health care utilization among women with congenital heart disease. Congenit Heart Dis. 2019;14:735‐744. - PubMed
-
- Gelson E, Curry R, Gatzoulis MA, et al. Effect of maternal heart disease on fetal growth. Obstet Gynecol. 2011;117:886‐891. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

