SARS-CoV2 wild type and mutant specific humoral and T cell immunity is superior after vaccination than after natural infection
- PMID: 35468147
- PMCID: PMC9037910
- DOI: 10.1371/journal.pone.0266701
SARS-CoV2 wild type and mutant specific humoral and T cell immunity is superior after vaccination than after natural infection
Abstract
Objective: We investigated blood samples from fully SARS-CoV2-vaccinated subjects and from previously positive tested patients up to one year after infection with SARS-CoV2, and compared short- and long-term T cell and antibody responses, with a special focus on the recently emerged delta variant (B.1.617.2).
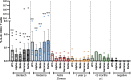
Methods and results: In 23 vaccinated subjects, we documented high anti-SARS-CoV2 spike protein receptor binding domain (RBD) antibody titers. Average virus neutralization by antibodies, assessed as inhibition of ACE2 binding to RBD, was 2.2-fold reduced for delta mutant vs. wild type (wt) RBD. The mean specific antibody titers were lower one year after natural infection than after vaccination; ACE2 binding to delta mutant vs. wt RBD was 1.65-fold reduced. In an additional group, omicron RBD binding was reduced compared to delta. Specific CD4+ T cell responses were measured after stimulation with peptides pools from wt, alpha, beta, gamma, or delta variant SARS-CoV2 spike proteins by flow cytometric intracellular cytokine staining. There was no significant difference in cytokine production of IFN-γ, TNF-α, or IL-2 between vaccinated subjects. T cell responses to wt or mutant SARS-CoV2 spike were significantly weaker after natural occurring infections compared to those in vaccinated individuals.
Conclusion: Antibody neutralisation of the delta mutant was reduced compared to wt, as assessed in a novel inhibition assay with a finger prick blood drop. Strong CD4 T cell responses were present against wt and mutant SARS-CoV2 variants, including the delta (B.1.617.2) strain, in fully vaccinated individuals, whereas they were partly weaker 1 year after natural infection. Hence, immune responses after vaccination are stronger compared to those after naturally occurring infection, pointing out the need of the vaccine to overcome the pandemic.
Conflict of interest statement
The fact that all authors are employees of the non-profit ISAR Bioscience Institute which formally acts as a biotech company does not alter our adherence to PLOS ONE policies on sharing data and materials. The authors or ISAR do not impose any restrictions on sharing of data and/or materials.
Figures







References
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

