Outcomes of relapsed/refractory diffuse large B-cell lymphoma and influence of chimaeric antigen receptor T trial eligibility criteria in second line-A population-based study of 736 patients
- PMID: 35468219
- PMCID: PMC9545648
- DOI: 10.1111/bjh.18197
Outcomes of relapsed/refractory diffuse large B-cell lymphoma and influence of chimaeric antigen receptor T trial eligibility criteria in second line-A population-based study of 736 patients
Abstract
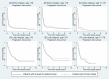
Several recently published trials investigate novel therapies for relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL). To estimate the benefit of these therapies in the real-world setting, comprehensive data on patients treated in clinical routine are needed. We report outcomes for 736 R/R DLBCL patients identified among all curatively treated DLBCL patients in Sweden in the period 2007-2014. Survival and associations with disease characteristics, second-line treatment and fulfilment of chimaeric antigen receptor (CAR) T-cell trial criteria were assessed. Median overall survival (OS) was 6.6 months (≤70 years 9.6 months, >70 years 4.9 months). Early relapse (≤12 months) was strongly associated with selection of less intensive treatment and poor survival. Among patients of at most 70 years of age, 63% started intensive second-line treatment and 34% received autologous stem cell transplantation (ASCT). Two-year OS among transplanted patients was 56% (early relapse ≤12 months 40%, late relapse >12 months 66%). A minority of patients 76 years (n = 178/506, 35%) fitted CAR T trial criteria. Median progression-free survival (PFS) for patients with early relapse fitting trial criteria was 4.8 months. In conclusion, most R/R DLBCL manifest early and are often ineligible for or cannot complete intensive regimens resulting in dismal survival. Real-world patients eligible for CAR T trials also did poorly, providing a benchmark for efficacy of novel therapies.
Keywords: clinical research; epidemiology; non-Hodgkin lymphoma; stem cell transplantation; tumour immunotherapy.
© 2022 The Authors. British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
El‐Galaly: previous employment by Roche Ltd (Basel) and speakers fee from Abbvie. Jerkeman: research support from Abbvie, AstraZeneca, Janssen, Gilead, BMS and Roche. Honoraria from Abbvie, AstraZeneca, Janssen, Novartis, Incyte, EUSApharma, Gilead, BMS and Roche. Sander: speaker’s fee from Roche and Sanofi. Smedby: research support from Janssen Pharmaceutical NV and Takeda. Harrysson, Eloranta, Ekberg, Andersson: no relevant conflicts to declare.
Figures




References
-
- Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, et al. Distinct types of diffuse large B‐cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–11. - PubMed
-
- Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large‐B‐cell lymphoma. N Engl J Med. 2002;346:1937–47. - PubMed
-
- Shipp MA, Ross KN, Tamayo P, Weng AP, Kutok JL, Aguiar RC, et al. Diffuse large B‐cell lymphoma outcome prediction by gene‐expression profiling and supervised machine learning. Nat Med. 2002;8:68–74. - PubMed
-
- Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large‐B‐cell lymphoma. N Engl J Med. 2002;346:235–42. - PubMed
-
- Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab‐CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B‐cell lymphoma. J Clin Oncol. 2006;24:3121–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

