Duration of treatment in oncology clinical trials: does the duration change when the same drug moves from the experimental arm to the control arm?
- PMID: 35468562
- PMCID: PMC9271471
- DOI: 10.1016/j.esmoop.2022.100480
Duration of treatment in oncology clinical trials: does the duration change when the same drug moves from the experimental arm to the control arm?
Abstract
Background: When a new drug comes to the market, the incentive for the sponsoring company is to maximize the treatment duration in order for the patient to reap the full therapeutic benefit of the product and achieve a positive trial result. We sought to enumerate instances when an already-approved oncology drug was used as a comparator for a newer drug seeking approval and compare the duration of treatment when it is used in the intervention arm to when it is used as a comparator.
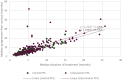
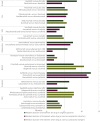
Patients and methods: In a cross-sectional analysis, we searched drug approval announcements for advanced, metastatic, or unresectable cancers between 2009 and 2020. We included studies reporting on an approved drug and studies reporting on when the same drug was used as a comparator for other drugs seeking Food and Drug Administration (FDA) approval. We examined median progression-free survival and duration of treatment for when the drug was initially approved and for when the drug was used as a comparator for other drugs that were seeking approval.
Results: Of the 23 instances when an approved drug was later used as a comparator against a newer drug seeking FDA approval, we found 11 instances (47.8%) where the drug, when used as a comparator arm, had a shorter duration of treatment than when it was used in the intervention arm. The median duration of treatment in the study initially testing the drug was 6.0 months (range: 2.2-12.7 months), whereas the median duration of treatment when the same drug was used as a comparator was 4.9 months (range: 1.7-12.0 months).
Conclusions: These results suggest that there is bias in how long a patient receives a given therapy, and this bias favors the newer therapy. Clinical trialists should seek to utilize methodology that reduces bias so that the relative efficacy of newer drugs can be objectively assessed.
Keywords: clinical trial; comparator; drug approval; duration of treatment; intervention; progression-free survival.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure VP: (research funding) Arnold Ventures, (royalties) Johns Hopkins Press, Medscape (honoraria) grand rounds/lectures from universities, medical centers, non-profits, professional societies, YouTube, and Substack. (Consulting) UnitedHealthcare. (Speaking fees) Evicore. (Other) plenary session podcast has patreon backers. All other authors have declared no conflicts of interest. Data sharing All data are publicly available on the FDA website and published in scientific journals.
Figures
References
-
- Lythgoe M.P., Krell J., Mahmoud S., Mills E.C., Vasudevan A., Savage P. Development and economic trends in anticancer drugs licensed in the UK from 2015 to 2019. Drug Discov Today. 2021;26(2):301–307. - PubMed
-
- Blumenthal G.M., Gong Y., Kehl K., et al. Analysis of time-to-treatment discontinuation of targeted therapy, immunotherapy, and chemotherapy in clinical trials of patients with non-small-cell lung cancer. Ann Oncol. 2019;30(5):830–838. - PubMed

