Functionality, satisfaction, and global impression of change with ubrogepant for the acute treatment of migraine in triptan insufficient responders: a post hoc analysis of the ACHIEVE I and ACHIEVE II randomized trials
- PMID: 35468729
- PMCID: PMC9036751
- DOI: 10.1186/s10194-022-01419-7
Functionality, satisfaction, and global impression of change with ubrogepant for the acute treatment of migraine in triptan insufficient responders: a post hoc analysis of the ACHIEVE I and ACHIEVE II randomized trials
Abstract
Background: Triptans are the first-line option for the acute treatment of migraine attacks; however, triptans are contraindicated in people with certain underlying cardiovascular risk factors and are associated with inadequate efficacy or poor tolerability in some individuals. Ubrogepant is an oral calcitonin gene-related peptide receptor antagonist approved for the acute treatment of migraine.
Methods: This post hoc analysis of the phase 3 ACHIEVE trials examined the impact of ubrogepant on the Functional Disability Scale (FDS), satisfaction with medication, and Patient Global Impression of Change (PGIC) in participants who were self-reported triptan insufficient responders (TIRs), defined as those who are unable to take triptans due to contraindications, tolerability issues, or insufficient efficacy. Responder definitions for the FDS, satisfaction measures, and PGIC were based on qualitative interpretation of the respective response options for the pooled ubrogepant 50 mg and placebo groups.
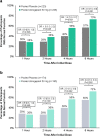
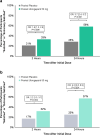
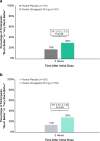
Results: In the pooled analysis population (n = 1799), 451 (25%) participants were TIRs, with most (80%) reporting insufficient efficacy with triptan use. A significantly higher proportion of TIRs treated with ubrogepant vs placebo reported being able to function normally from 2 to 8 h post dose (P < 0.05). Notably, significance was demonstrated at the time of the primary outcome assessments (2 h post dose), where rates of normal function were 38% for ubrogepant vs 29% for placebo (P = 0.048). A greater proportion of TIRs in the ubrogepant arm vs the placebo arm were satisfied with treatment at 2 (33% vs 21%, P = 0.006) and 24 h (58% vs 28%, P < 0.001) and indicated that their migraine improved at 2 h vs placebo (30% vs 18%, P = 0.006). Results were generally similar in the insufficient efficacy subpopulation of TIRs as in the overall TIRs group. Ubrogepant was safe and well tolerated in TIRs, with no new safety signals identified.
Conclusions: In people with migraine who are TIRs, individuals treated with ubrogepant had favorable 2-h outcomes, as measured by the FDS, satisfaction with medication, and PGIC, compared with placebo.
Trial registration: ClinicalTrials.gov: NCT02828020 (ACHIEVE I), registered July 11, 2016; NCT02867709 (ACHIEVE II), registered August 16, 2016.
Keywords: ACHIEVE; Calcitonin gene–related peptide receptor antagonist; Functionality; Migraine; Patient-reported outcomes; Satisfaction; Triptan.
© 2022. The Author(s).
Conflict of interest statement
RBL has received research support from the National Institutes of Health, the FDA, and the National Headache Foundation. He serves as consultant, advisory board member, or has received honoraria or research support from AbbVie/Allergan, Amgen, Biohaven, Dr. Reddy’s Laboratories (Promius), electroCore, Eli Lilly, GlaxoSmithKline, Lundbeck, Merck, Novartis, Teva, Vector, and Vedanta Research. He receives royalties from
RBHS reports honoraria for advisory boards from Impel, Supernus, and Teva, and grants for research support from Amgen and Eli Lilly.
DAR received research support from Allergan and Amgen, and served as a consultant for AbbVie, Allergan, and Amgen.
DWD reports the following conflicts: Consulting: Amgen, Atria, Cerecin, Cooltech, Ctrl M, Allergan, Abbvie, Biohaven, GSK, Lundbeck, Eli Lilly, Novartis, Impel, Satsuma, Theranica, WL Gore, Genentech, Nocira, Perfood, Praxis, AYYA Biosciences, Revance. Honoraria: American Academy of Neurology, Headache Cooperative of the Pacific, MF Med Ed Research, Biopharm Communications, CEA Group Holding Company (Clinical Education Alliance LLC), Teva (speaking), Amgen (speaking), Eli Lilly (speaking), Lundbeck (speaking), Vector Psychometric Group, Clinical Care Solutions, CME Outfitters, Curry Rockefeller Group, DeepBench, Global Access Meetings, KLJ Associates, Academy for Continued Healthcare Learning, Majallin LLC, Medlogix Communications, Medica Communications LLC, MJH Lifesciences, Miller Medical Communications, WebMD Health/Medscape, Wolters Kluwer, Oxford University Press, Cambridge University Press. Non-profit board membership: American Brain Foundation, American Migraine Foundation, ONE Neurology, Precon Health Foundation, International Headache Society Global Patient Advocacy Coalition, Atria Health Collaborative, Domestic Violence HOPE Foundation/Panfila. Research Support: Department of Defense, National Institutes of Health, Henry Jackson Foundation, Sperling Foundation, American Migraine Foundation, Patient Centered Outcomes Research Institute (PCORI). Stock Options/Shareholder/Patents/Board of Directors: Ctrl M (options), Aural analytics (options), ExSano (options), Palion (options), Healint (options), Theranica (options), Second Opinion/Mobile Health (options), Epien (options/Board), Nocira (options), Matterhorn (shares/Board), Ontologics (shares/Board), King-Devick Technologies (options/Board), Precon Health (options/Board), AYYA Biosciences (options), Axon Therapeutics (options/Board), Cephalgia Group (options/Board), Atria Health (options/employee). Patent 17189376.1–1466:vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis.
ARS is an employee of AbbVie, and may hold AbbVie stock.
SZ and JEL were employees of AbbVie at the time of study conduct and may hold AbbVie stock.
Figures

References
-
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. - DOI - PMC - PubMed
-
- Headache Classification Committee of the International Headache Society (2018) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38:1–211 - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

