The Determining Effective Testing in Emergency Departments and Care Coordination on Treatment Outcomes (DETECT) for Hepatitis C (Hep C) Screening Trial: rationale and design of a multi-center pragmatic randomized clinical trial of hepatitis C screening in emergency departments
- PMID: 35468807
- PMCID: PMC9036509
- DOI: 10.1186/s13063-022-06265-1
The Determining Effective Testing in Emergency Departments and Care Coordination on Treatment Outcomes (DETECT) for Hepatitis C (Hep C) Screening Trial: rationale and design of a multi-center pragmatic randomized clinical trial of hepatitis C screening in emergency departments
Abstract
Background: Early identification of HCV is a critical health priority, especially now that treatment options are available to limit further transmission and provide cure before long-term sequelae develop. Emergency departments (EDs) are important clinical settings for HCV screening given that EDs serve many at-risk patients who do not access other forms of healthcare. In this article, we describe the rationale and design of The Determining Effective Testing in Emergency Departments and Care Coordination on Treatment Outcomes (DETECT) for Hepatitis C (Hep C) Screening Trial.
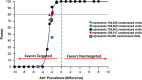
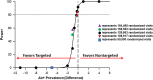
Methods: The DETECT Hep C Screening Trial is a multi-center prospective pragmatic randomized two-arm parallel-group superiority trial to test the comparative effectiveness of nontargeted and targeted HCV screening in the ED with a primary hypothesis that nontargeted screening is superior to targeted screening when identifying newly diagnosed HCV. This trial will be performed in the EDs at Denver Health Medical Center (Denver, CO), Johns Hopkins Hospital (Baltimore, MD), and the University of Mississippi Medical Center (Jackson, MS), sites representing approximately 225,000 annual adult visits, and designed using the PRECIS-2 framework for pragmatic trials. When complete, we will have enrolled a minimum of 125,000 randomized patient visits and have performed 13,965 HCV tests. In Denver, the Screening Trial will serve as a conduit for a distinct randomized comparative effectiveness trial to evaluate linkage-to-HCV care strategies. All sites will further contribute to embedded observational studies to assess cost effectiveness, disparities, and social determinants of health in screening, linkage-to-care, and treatment for HCV.
Discussion: When complete, The DETECT Hep C Screening Trial will represent the largest ED-based pragmatic clinical trial to date and all studies, in aggregate, will significantly inform how to best perform ED-based HCV screening.
Trial registration: ClinicalTrials.gov ID: NCT04003454 . Registered on 1 July 2019.
Keywords: Clinical trial; Comparative effectiveness; Emergency department; HCV; Hepatitis C; Implementation; Methods; Pragmatic trial; Prevention; Randomized trial; Screening; Testing.
© 2022. The Author(s).
Conflict of interest statement
JSH has received grant funding from the Centers for Disease Control and Prevention (CDC) (R01CE003006). SER has received programmatic funding from Gilead Sciences, Inc. KFK receives salary support via grant funding from Gilead Sciences, Inc. JRM has received grant funding from the National Institute on Drug Abuse (NIDA) (P30DA040500). EMG serves as site principal investigator for clinical trials sponsored by Cepheid, Gilead Science, and Viiv Healthcare. MSL and DAEW receive investigator-initiated grant support paid to the institution from Gilead Sciences. All other authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

