Impaired signaling for neuromuscular synaptic maintenance is a feature of Motor Neuron Disease
- PMID: 35468848
- PMCID: PMC9040261
- DOI: 10.1186/s40478-022-01360-5
Impaired signaling for neuromuscular synaptic maintenance is a feature of Motor Neuron Disease
Abstract
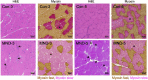
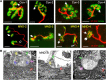
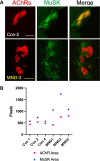
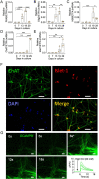
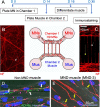
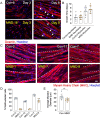
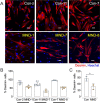
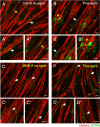
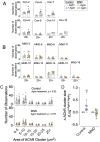
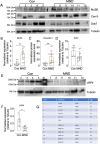
A central event in the pathogenesis of motor neuron disease (MND) is the loss of neuromuscular junctions (NMJs), yet the mechanisms that lead to this event in MND remain to be fully elucidated. Maintenance of the NMJ relies upon neural agrin (n-agrin) which, when released from the nerve terminal, activates the postsynaptic Muscle Specific Kinase (MuSK) signaling complex to stabilize clusters of acetylcholine receptors. Here, we report that muscle from MND patients has an increased proportion of slow fibers and muscle fibers with smaller diameter. Muscle cells cultured from MND biopsies failed to form large clusters of acetylcholine receptors in response to either non-MND human motor axons or n-agrin. Furthermore, levels of expression of MuSK, and MuSK-complex components: LRP4, Caveolin-3, and Dok7 differed between muscle cells cultured from MND patients compared to those from non-MND controls. To our knowledge, this is the first time a fault in the n-agrin-LRP4-MuSK signaling pathway has been identified in muscle from MND patients. Our results highlight the n-agrin-LRP4-MuSK signaling pathway as a potential therapeutic target to prolong muscle function in MND.
Keywords: ALS; Acetylcholine receptors; Agrin; Amyotrophic Lateral Sclerosis; Motor neurons; MuSK; Neuromuscular junction.
© 2022. The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

