Unsupervised machine learning improves risk stratification in newly diagnosed multiple myeloma: an analysis of the Spanish Myeloma Group
- PMID: 35468898
- PMCID: PMC9038663
- DOI: 10.1038/s41408-022-00647-z
Unsupervised machine learning improves risk stratification in newly diagnosed multiple myeloma: an analysis of the Spanish Myeloma Group
Abstract
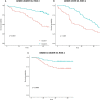
The International Staging System (ISS) and the Revised International Staging System (R-ISS) are commonly used prognostic scores in multiple myeloma (MM). These methods have significant gaps, particularly among intermediate-risk groups. The aim of this study was to improve risk stratification in newly diagnosed MM patients using data from three different trials developed by the Spanish Myeloma Group. For this, we applied an unsupervised machine learning clusterization technique on a set of clinical, biochemical and cytogenetic variables, and we identified two novel clusters of patients with significantly different survival. The prognostic precision of this clusterization was superior to those of ISS and R-ISS scores, and appeared to be particularly useful to improve risk stratification among R-ISS 2 patients. Additionally, patients assigned to the low-risk cluster in the GEM05 over 65 years trial had a significant survival benefit when treated with VMP as compared with VTD. In conclusion, we describe a simple prognostic model for newly diagnosed MM whose predictions are independent of the ISS and R-ISS scores. Notably, the model is particularly useful in order to re-classify R-ISS score 2 patients in 2 different prognostic subgroups. The combination of ISS, R-ISS and unsupervised machine learning clusterization brings a promising approximation to improve MM risk stratification.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that no support was provided in the forms of grants and/or equipment and grants for the development of this study. M.-V.M. has received honoraria for lectures and participation in advisory boards from Janssen, Celgene-BMS, Amgen, Takeda, Abbvie, GSK, Adaptive, Roche, Seattle Genetics, Pfizer, and Regeneron. A.M.O reports honoraria for lectures and participation in advisory boards from Janssen, Takeda, Abbvie, Amgen, Novartis, Gilead and AstraZeneca; research grants from Roche, Pfizer and Celgene-BMS and funds for conference organization from Jassen, Takeda, Abbvie, Amgen, Novartis, Gilead, Roche, Bristol-Myers-Squibb, Glaxo-Smith-Klyne, Incyte and Pfizer. M.S.G.P. has received honoraria for lectures and participation in advisory boards from Janssen, Amgen, Celgene-BMS, Takeda, Sanofi and GSK. J.A.D.A.: has received honoraria for lectures from Abbvie and Janssen. L.R. reports Honoraria from Janssen, BMS-Celgene, Amgen, TAkeda, Sanofi, GSK and Karyopharm. A.O. reports advisory board fees from Bristol Myers Squibb, Janssen, and Amgen. A.I.T.: no COIs to disclose. L.P.: no COIs to disclose. M.T.H.: no COIs to disclose. E.B.: no COIs to disclose. M.G. has received honoraria from Janssen-Cilag and Celgene. M.J.B. declares honoraria from lectures and advisory boards from Janssen, BMS/Celgene, Amgen, Takeda, and GSK. J.d.l.R. has served as a consultant and provided expert testimony within the past 2 years for Amgen, Celgene, Takeda, Janssen, and Sanofi. A.L. has received honoraria for advisory boards from Celgene, Amgen, and Janssen. A.S. reports honoraria from Takeda, BMS, MSD, Sanofi, Roche, Novartis y Janssen; consultancy: Takeda, BMS, Novartis, Jansser, Gilead, Sanofi, GSK; Speaker’s bureau: Takeda; Research Support: Takeda. M.J.C.: no COIs to disclose. R.R. has received honoraria for lectures and participation in advisory boards from Becton-Dickinson, Celgene, Janssen, Sanofi and Binding Site. J.M.L. has received honoraria for lectures and participation in advisory boards from Janssen, Celgene-BMS, Amgen, Takeda, Abbvie, GSK, Adaptive, Roche, Pfizer, and Astellas, Incyte. He has received research grants from BMS, Roche, Astellas and Janssen. J.B. reports honoraria from Janssen, Celgene, Takeda, Amgen and Oncopeptides. J.J.L. reports a consulting or advisory role for Celgene, Takeda, Amgen, Janssen and Sanofi and travel accommodations and expenses from Celgene. J.F.S.M. reports a consultancy or advisory role for AbbVie, Amgen, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Karyopharm, MSD, Novartis, Roche, Sanofi, SecuraBio and Takeda.
Figures



References
-
- Schavgoulidze A, Lauwers-Cances V, Perrot A, Avet-Loiseau4 H, Corre J. The discriminatory ability of the R ÍSS is equivalent to ISS in a large cohort of newly diagnosed Multiple Myeloma patients. 62 ASH Annual Meeting. Abstract 1338. https://ash.confex.com/ash/2020/webprogram/Paper136996.html
-
- Biccler JL, Eloranta S, de Nully Brown P, Frederiksen H, Jerkeman M, Jørgensen J, et al. Optimizing outcome prediction in diffuse large B-cell lymphoma by use of machine learning and nationwide lymphoma registries: a nordic lymphoma group study. JCO Clin Cancer Inf. 2018;2:1–13. doi: 10.1200/CCI.18.00025. - DOI - PubMed

