Transcription factors AP-2α and AP-2β regulate distinct segments of the distal nephron in the mammalian kidney
- PMID: 35468900
- PMCID: PMC9038906
- DOI: 10.1038/s41467-022-29644-3
Transcription factors AP-2α and AP-2β regulate distinct segments of the distal nephron in the mammalian kidney
Abstract
Transcription factors AP-2α and AP-2β have been suggested to regulate the differentiation of nephron precursor populations towards distal nephron segments. Here, we show that in the adult mammalian kidney AP-2α is found in medullary collecting ducts, whereas AP-2β is found in distal nephron segments except for medullary collecting ducts. Inactivation of AP-2α in nephron progenitor cells does not affect mammalian nephrogenesis, whereas its inactivation in collecting ducts leads to defects in medullary collecting ducts in the adult. Heterozygosity for AP-2β in nephron progenitor cells leads to progressive distal convoluted tubule abnormalities and β-catenin/mTOR hyperactivation that is associated with renal fibrosis and cysts. Complete loss of AP-2β in nephron progenitor cells caused an absence of distal convoluted tubules, renal cysts, and fibrosis with β-catenin/mTOR hyperactivation, and early postnatal death. Thus, AP-2α and AP-2β have non-redundant distinct spatiotemporal functions in separate segments of the distal nephron in the mammalian kidney.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


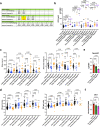
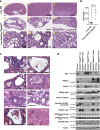

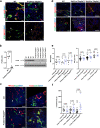
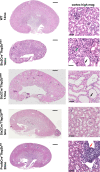

Similar articles
-
AP-2β/KCTD1 Control Distal Nephron Differentiation and Protect against Renal Fibrosis.Dev Cell. 2020 Aug 10;54(3):348-366.e5. doi: 10.1016/j.devcel.2020.05.026. Epub 2020 Jun 17. Dev Cell. 2020. PMID: 32553120 Free PMC article.
-
β-catenin regulates the formation of multiple nephron segments in the mouse kidney.Sci Rep. 2019 Nov 4;9(1):15915. doi: 10.1038/s41598-019-52255-w. Sci Rep. 2019. PMID: 31685872 Free PMC article.
-
The elasmobranch kidney. II. Sequence and structure of the nephrons.Anat Embryol (Berl). 1985;173(2):163-86. doi: 10.1007/BF00316299. Anat Embryol (Berl). 1985. PMID: 4083520
-
Renal potassium transport: contributions of individual nephron segments and populations.Am J Physiol. 1978 Dec;235(6):F515-27. doi: 10.1152/ajprenal.1978.235.6.F515. Am J Physiol. 1978. PMID: 367178 Review.
-
Ammonia transport in the mammalian kidney.Am J Physiol. 1985 Apr;248(4 Pt 2):F459-71. doi: 10.1152/ajprenal.1985.248.4.F459. Am J Physiol. 1985. PMID: 3885755 Review.
Cited by
-
Transcription factor Ap2b regulates the mouse autosomal recessive polycystic kidney disease genes, Pkhd1 and Cys1.Front Mol Biosci. 2023 Jan 12;9:946344. doi: 10.3389/fmolb.2022.946344. eCollection 2022. Front Mol Biosci. 2023. PMID: 36710876 Free PMC article.
-
(Zebra)fishing for nephrogenesis genes.Tissue Barriers. 2024 Apr 2;12(2):2219605. doi: 10.1080/21688370.2023.2219605. Epub 2023 May 31. Tissue Barriers. 2024. PMID: 37254823 Free PMC article. Review.
-
The regulatory role of AP-2β in monoaminergic neurotransmitter systems: insights on its signalling pathway, linked disorders and theragnostic potential.Cell Biosci. 2022 Sep 8;12(1):151. doi: 10.1186/s13578-022-00891-7. Cell Biosci. 2022. PMID: 36076256 Free PMC article. Review.
-
AP-2α/AP-2β transcription factors are key regulators of epidermal homeostasis.bioRxiv [Preprint]. 2023 Dec 4:2023.12.03.569763. doi: 10.1101/2023.12.03.569763. bioRxiv. 2023. Update in: J Invest Dermatol. 2024 Jul;144(7):1505-1521.e12. doi: 10.1016/j.jid.2023.12.017. PMID: 38105942 Free PMC article. Updated. Preprint.
-
AP-2α/AP-2β Transcription Factors Are Key Regulators of Epidermal Homeostasis.J Invest Dermatol. 2024 Jul;144(7):1505-1521.e12. doi: 10.1016/j.jid.2023.12.017. Epub 2024 Jan 17. J Invest Dermatol. 2024. PMID: 38237728 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

