Inotuzumab ozogamicin as single agent in pediatric patients with relapsed and refractory acute lymphoblastic leukemia: results from a phase II trial
- PMID: 35468945
- PMCID: PMC9162924
- DOI: 10.1038/s41375-022-01576-3
Inotuzumab ozogamicin as single agent in pediatric patients with relapsed and refractory acute lymphoblastic leukemia: results from a phase II trial
Abstract
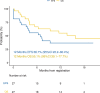
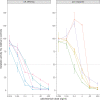
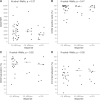
Inotuzumab Ozogamicin is a CD22-directed antibody conjugated to calicheamicin, approved in adults with relapsed or refractory (R/R) B cell acute lymphoblastic leukemia (BCP-ALL). Patients aged 1-18 years, with R/R CD22 + BCP-ALL were treated at the RP2D of 1.8 mg/m2. Using a single-stage design, with an overall response rate (ORR) ≤ 30% defined as not promissing and ORR > 55% as expected, 25 patients needed to be recruited to achieve 80% power at 0.05 significance level. Thirty-two patients were enrolled, 28 were treated, 27 were evaluable for response. The estimated ORR was 81.5% (95%CI: 61.9-93.7%), and 81.8% (18/22) of the responding subjects were minimal residual disease (MRD) negative. The study met its primary endpoint. Median follow up of survivors was 16 months (IQR: 14.49-20.07). One year Event Free Survival was 36.7% (95% CI: 22.2-60.4%), and Overall Survival was 55.1% (95% CI: 39.1-77.7%). Eighteen patients received consolidation (with HSCT and/or CAR T-cells therapy). Sinusoidal obstructive syndrome (SOS) occurred in seven patients. MRD negativity seemed correlated to calicheamicin sensitivity in vitro, but not to CD22 surface expression, saturation, or internalization. InO was effective in this population. The most relevant risk was the occurrence of SOS, particularly when InO treatment was followed by HSCT.
© 2022. The Author(s).
Conflict of interest statement
EP: received support for the present manuscript from the Princes Maxima Center. NM: received support for the present manuscript from the Princes Maxima Center. EB: received support for the present manuscript from the Princes Maxima Center. YJ: received support for the present manuscript from the Princes Maxima Center. BS: is Pfizer Inc Employee and Pfizer Inc stock holder. YC: is Pfizer Inc Employee and Pfizer Inc stock holder. BV: is in the Advisory Board for DSMB SeluDex Study. SR: is in the advisory boards of Novartis, Celgene/Bristol-Myers, Servier/Cellectis Kite and Amgen from which received travel expenses reimbursement, and is Co-chair of the Leukemia Group of the Spanish Pediatric Hematology and Oncology Society (SEHOP) (not paid), and National Representative of Spain in the I-BFM Study Group of Childhood Leukemia (not paid). CR (Claudia Rössig): received consulting fees from Amgen, Pfizer, BMS, Novartis, Celgene, Roche. CR: received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Jazz pharmaceuticals, Servier, Amgen, and support for attending meetings and/or travel from Jazz pharmaceuticals, Servier, Amgen, Gilead. GE: received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Servier. FJBS: received consulting fees from Bayer, Agmen Roche Genentech, EusaPharma, and is speaker at Symposia for Roche Genentech. YB: received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Servier, Sanofi Genzymea and financial support for attending meetings from Jazz pharmaceuticals and Servier. DR: received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Cerus. LV: received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Agmen and financial support for attending meetings and/or travel from Interlabo. AvS: received consulting fees from Amgen, Novartis and Jazz Pharmaceuticals, and payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Novartis and Miltenyi. FL: received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Amgen, Miltenyi, Bellicum, Medac, Neovii, BluebirdBio, and Gilead. CMZ: received financial support for the present manuscript from Pfizer Inc, grants or contracts from any entity from BMS, Abbvie and JAZZ pharmaceuticals, consulting fees from Sanofi, Novartis, Roche, Pfizer Inc and Incyte, financial support for attending meetings and/or travel from JAZZ pharmaceuticals.
Figures
References
-
- Henze G, Stackelberg A V, Eckert C ALL-REZ BFM–The consecutive trials for children with relapsed acute lymphoblastic leukemia. Klin Padiatr. 2013;225:S73–8 (Supplement 1). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

