Disparities in COVID-19 Monoclonal Antibody Delivery: a Retrospective Cohort Study
- PMID: 35469360
- PMCID: PMC9037582
- DOI: 10.1007/s11606-022-07603-4
Disparities in COVID-19 Monoclonal Antibody Delivery: a Retrospective Cohort Study
Abstract
Background: Disparities in access to anti-SARS-CoV-2 monoclonal antibodies have not been well characterized.
Objective: We sought to explore the impact of race/ethnicity as a social construct on monoclonal antibody delivery.
Design/patients: Following implementation of a centralized infusion program at a large academic healthcare system, we reviewed a random sample of high-risk ambulatory adult patients with COVID-19 referred for monoclonal antibody therapy.
Main measures: We examined the relationship between treatment delivery, race/ethnicity, and other demographics using descriptive statistics, binary logistic regression, and spatial analysis.
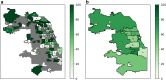
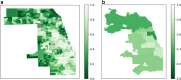
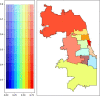
Key results: There was no significant difference in racial composition between patients who did (n = 25) and patients who did not (n = 378) decline treatment (p = 0.638). Of patients who did not decline treatment, 64.8% identified as White, 14.8% as Hispanic/Latinx, and 11.1% as Black. Only 44.6% of Hispanic/Latinx and 31.0% of Black patients received treatment compared to 64.1% of White patients (OR 0.45, 95% CI 0.25-0.81, p = 0.008, and OR 0.25, 95% CI 0.12-0.50, p < 0.001, respectively). In multivariable analysis including age, race, insurance status, non-English primary language, county Social Vulnerability Index, illness severity, and total number of comorbidities, associations between receiving treatment and Hispanic/Latinx or Black race were no longer statistically significant (AOR 1.32, 95% CI 0.69-2.53, p = 0.400, and AOR 1.34, 95% CI 0.64-2.80, p = 0.439, respectively). However, patients who were uninsured or whose primary language was not English were less likely to receive treatment (AOR 0.16, 95% CI 0.03-0.88, p = 0.035, and AOR 0.37, 95% CI 0.15-0.90, p = 0.028, respectively). Spatial analysis suggested decreased monoclonal antibody delivery to Cook County patients residing in socially vulnerable communities.
Conclusions: High-risk ambulatory patients with COVID-19 who identified as Hispanic/Latinx or Black were less likely to receive monoclonal antibody therapy in univariate analysis, a finding not explained by patient refusal. Multivariable and spatial analyses suggested insurance status, language, and social vulnerability contributed to racial disparities.
Keywords: COVID-19; SARS-CoV-2; Social Vulnerability Index; monoclonal antibody; racial/ethnic disparities.
© 2022. The Author(s), under exclusive licence to Society of General Internal Medicine.
Conflict of interest statement
V. Stosor reports research support, paid to Northwestern University, from Eli Lilly and Company. M. G. Ison reports research support, paid to Northwestern University, from GlaxoSmithKline and Regeneron.
Figures

References
-
- Eli Lilly and Company. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Bamlanivimab and Etesevimab. U.S. Food and Drug Administration; 2021 [cited 2021 Oct 1]. Available from: https://www.fda.gov/media/145802/download.
-
- Regeneron Pharmaceuticals, Inc. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of REGEN-COVTM (casirivimab and imdevimab). U.S. Food and Drug Administration; 2021 [cited 2021 Oct 1]. Available from: https://www.fda.gov/media/145611/download.
-
- GlaxoSmithKline. Fact Sheet for Health Care Providers Emergency Use Authorization (EUA) of Sotrovimab. U.S. Food and Drug Administration; 2021 [cited 2021 Oct 1]. Available from: https://www.fda.gov/media/149534/download.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

