Angiopoietin 2 Is Associated with Vascular Necroptosis Induction in Coronavirus Disease 2019 Acute Respiratory Distress Syndrome
- PMID: 35469796
- PMCID: PMC9027298
- DOI: 10.1016/j.ajpath.2022.04.002
Angiopoietin 2 Is Associated with Vascular Necroptosis Induction in Coronavirus Disease 2019 Acute Respiratory Distress Syndrome
Abstract
Vascular injury is a well-established, disease-modifying factor in acute respiratory distress syndrome (ARDS) pathogenesis. Recently, coronavirus disease 2019 (COVID-19)-induced injury to the vascular compartment has been linked to complement activation, microvascular thrombosis, and dysregulated immune responses. This study sought to assess whether aberrant vascular activation in this prothrombotic context was associated with the induction of necroptotic vascular cell death. To achieve this, proteomic analysis was performed on blood samples from COVID-19 subjects at distinct time points during ARDS pathogenesis (hospitalized at risk, N = 59; ARDS, N = 31; and recovery, N = 12). Assessment of circulating vascular markers in the at-risk cohort revealed a signature of low vascular protein abundance that tracked with low platelet levels and increased mortality. This signature was replicated in the ARDS cohort and correlated with increased plasma angiopoietin 2 levels. COVID-19 ARDS lung autopsy immunostaining confirmed a link between vascular injury (angiopoietin 2) and platelet-rich microthrombi (CD61) and induction of necrotic cell death [phosphorylated mixed lineage kinase domain-like (pMLKL)]. Among recovery subjects, the vascular signature identified patients with poor functional outcomes. Taken together, this vascular injury signature was associated with low platelet levels and increased mortality and can be used to identify ARDS patients most likely to benefit from vascular targeted therapies.
Copyright © 2022 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

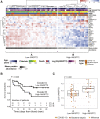
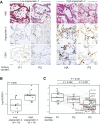
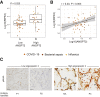
Comment in
-
Dynamic Angiopoietin-2 Serum Level as Endothelial Damage Marker and Potential Therapeutic Target.Am J Pathol. 2022 Sep;192(9):1336-1337. doi: 10.1016/j.ajpath.2022.05.011. Am J Pathol. 2022. PMID: 36064255 Free PMC article. No abstract available.
References
-
- Ziegler T., Horstkotte J., Schwab C., Pfetsch V., Weinmann K., Dietzel S., Rohwedder I., Hinkel R., Gross L., Lee S., Hu J., Soehnlein O., Franz W.M., Sperandio M., Pohl U., Thomas M., Weber C., Augustin H.G., Fässler R., Deutsch U., Kupatt C. Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. J Clin Invest. 2013;123:3436–3445. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

