Loss of UCP1 function augments recruitment of futile lipid cycling for thermogenesis in murine brown fat
- PMID: 35470094
- PMCID: PMC9097615
- DOI: 10.1016/j.molmet.2022.101499
Loss of UCP1 function augments recruitment of futile lipid cycling for thermogenesis in murine brown fat
Abstract
Objective: Classical ATP-independent non-shivering thermogenesis enabled by uncoupling protein 1 (UCP1) in brown adipose tissue (BAT) is activated, but not essential for survival, in the cold. It has long been suspected that futile ATP-consuming substrate cycles also contribute to thermogenesis and can partially compensate for the genetic ablation of UCP1 in mouse models. Futile ATP-dependent thermogenesis could thereby enable survival in the cold even when brown fat is less abundant or missing.
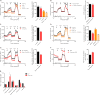
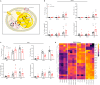
Methods: In this study, we explore different potential sources of UCP1-independent thermogenesis and identify a futile ATP-consuming triglyceride/fatty acid cycle as the main contributor to cellular heat production in brown adipocytes lacking UCP1. We uncover the mechanism on a molecular level and pinpoint the key enzymes involved using pharmacological and genetic interference.
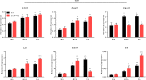
Results: ATGL is the most important lipase in terms of releasing fatty acids from lipid droplets, while DGAT1 accounts for the majority of fatty acid re-esterification in UCP1-ablated brown adipocytes. Furthermore, we demonstrate that chronic cold exposure causes a pronounced remodeling of adipose tissues and leads to the recruitment of lipid cycling capacity specifically in BAT of UCP1-knockout mice, possibly fueled by fatty acids from white fat. Quantification of triglyceride/fatty acid cycling clearly shows that UCP1-ablated animals significantly increase turnover rates at room temperature and below.
Conclusion: Our results suggest an important role for futile lipid cycling in adaptive thermogenesis and total energy expenditure.
Keywords: Brown adipose tissue; Fatty acids; Futile substrate cycle; Lipolysis; Re-esterification; UCP1-independent thermogenesis.
Copyright © 2022 The Author(s). Published by Elsevier GmbH.. All rights reserved.
Figures




References
-
- Newsholme E.A., Crabtree B. Substrate cycles in metabolic regulation and in heat generation. Biochem Soc Symp. 1976;(41):61–109. - PubMed
-
- Newsholme E.A. Substrate cycles: their metabolic, energetic and thermic consequences in man. Biochem Soc Symp. 1978;(43):183–205. - PubMed
-
- Newsholme E.A., Arch J.R., Brooks B., Surholt B. The role of substrate cycles in metabolic regulation. Biochem Soc Trans. 1983;11(1):52–56. - PubMed
-
- Newsholme E.A., Board Sounding. A possible metabolic basis for the control of body weight. N Engl J Med. 1980;302(7):400–405. - PubMed
-
- Newsholme E.A., Challiss R.A.J., Crabtree B. Substrate cycles: their role in improving sensitivity in metabolic control. Trends in Biochemical Sciences. 1984;9(6):277–280.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

