Integrated continuous biomanufacturing on pilot scale for acid-sensitive monoclonal antibodies
- PMID: 35470430
- PMCID: PMC9541590
- DOI: 10.1002/bit.28120
Integrated continuous biomanufacturing on pilot scale for acid-sensitive monoclonal antibodies
Abstract
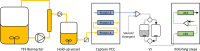
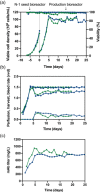
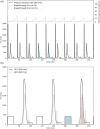
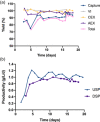
In this study, we demonstrated the first, to our knowledge, integrated continuous bioprocess (ICB) designed for the production of acid-sensitive monoclonal antibodies, prone to aggregate at low pH, on pilot scale. A high cell density perfusion culture, stably maintained at 100 × 106 cells/ml, was integrated with the downstream process, consisting of a capture step with the recently developed Protein A ligand, ZCa ; a solvent/detergent-based virus inactivation; and two ion-exchange chromatography steps. The use of a mild pH in the downstream process makes this ICB suitable for the purification of acid-sensitive monoclonal antibodies. Integration and automation of the downstream process were achieved using the Orbit software, and the same equipment and control system were used in initial small-scale trials and the pilot-scale downstream process. High recovery yields of around 90% and a productivity close to 1 g purified antibody/L/day were achieved, with a stable glycosylation pattern and efficient removal of impurities, such as host cell proteins and DNA. Finally, negligible levels of antibody aggregates were detected owing to the mild conditions used throughout the process. The present work paves the way for future industrial-scale integrated continuous biomanufacturing of all types of antibodies, regardless of acid stability.
Keywords: Chinese Hamster Ovary cells; ZCa ligand; antibody aggregation; antibody manufacturing; continuous chromatography; integrated continuous bioprocess; perfusion culture.
© 2022 The Authors. Biotechnology and Bioengineering published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: SH has filed a patent application regarding the novel ZCa domain.
Figures
References
-
- Chotteau, V. (2015). Perfusion processes. In Al‐Rubeai M. (Ed.), Animal cell culture (pp. 407–443). Springer.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

