Impact of adolescent intermittent ethanol exposure in male and female rats on social drinking and neuropeptide gene expression
- PMID: 35470441
- PMCID: PMC9247011
- DOI: 10.1111/acer.14847
Impact of adolescent intermittent ethanol exposure in male and female rats on social drinking and neuropeptide gene expression
Abstract
Background: Alcohol use during adolescence can alter maturational changes that occur in brain regions associated with social and emotional responding. Our previous studies have shown that adult male, but not female rats demonstrate social anxiety-like alterations and enhanced sensitivity to ethanol-induced social facilitation following adolescent intermittent ethanol exposure (AIE). These consequences of AIE may influence adult social drinking in a sex-specific manner.
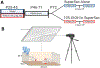
Methods: To test the effects of AIE on social drinking, male and female Sprague-Dawley rats exposed to water or ethanol (0 or 4 g/kg, intragastrically, every other day, between postnatal day [P] 25 and 45) were tested as adults (P72-83) in a social drinking paradigm (30-minute access to a 10% ethanol solution in supersac or supersac alone in groups of three same-sex littermates across two 4-day cycles separated by 4 days off). Social behavior was assessed during the last drinking session, along with assessment of oxytocin (OXT), oxytocin receptor (OXTR), vasopressin (AVP), and vasopressin receptors 1a and 1b (AVPR1a, AVPR1b) in the hypothalamus and lateral septum.
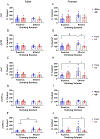
Results: Males exposed to AIE consumed more ethanol than water-exposed controls during the second drinking cycle, whereas AIE did not affect supersac intake in males. AIE-exposed females consumed less ethanol and more supersac than water-exposed controls. Water-exposed females drinking ethanol showed more social investigation and significantly higher hypothalamic OXTR, AVP, and AVPR1b gene expression than their counterparts ingesting supersac and AIE females drinking ethanol. In males, hypothalamic AVPR1b gene expression was affected by drinking solution, with significantly higher expression evident in males drinking ethanol than those consuming supersac.
Conclusions: Collectively, these findings provide new evidence regarding sex-specific effects of AIE on social drinking and suggest that the hypothalamic OXT and AVP systems are implicated in the effects of ingested ethanol on social behavior in a sex- and adolescent-exposure-dependent manner.
Keywords: adolescent intermittent ethanol exposure; oxytocin; sex differences; social drinking; vasopressin.
© 2022 by the Research Society on Alcoholism.
Figures


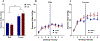

References
-
- Amodeo LR, Wills DN, Sanchez-Alavez M, Nguyen W, Conti B, & Ehlers CL (2018). Intermittent voluntary ethanol consumption combined with ethanol vapor exposure during adolescence increases drinking and alters other behaviors in adulthood in female and male rats. Alcohol, 73, 57–66. 10.1016/j.alcohol.2018.04.003 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

