Early predictors of corticosteroid response in acute severe autoimmune hepatitis: a nationwide multicenter study
- PMID: 35470447
- PMCID: PMC9324977
- DOI: 10.1111/apt.16926
Early predictors of corticosteroid response in acute severe autoimmune hepatitis: a nationwide multicenter study
Abstract
Background and aims: To assess whether corticosteroids improve prognosis in patients with AS-AIH, and to identify factors at therapy initiation and during therapy predictive of the response to corticosteroids.
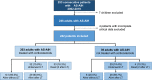
Methods: This was a retrospective cohort study including all patients with AS-AIH admitted to 13 tertiary centres from January 2002 to January 2019. The composite primary outcome was death or liver transplantation within 90 days of admission. Kaplan-Meier and Cox regression methods were used for data analysis.
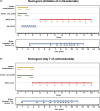
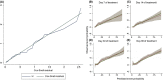
Results: Of 242 consecutive patients enrolled (mean age [SD] 49.7 [16.8] years), 203 received corticosteroids. Overall 90-day transplant-free survival was 61.6% (95% confidence interval [CI] 55.4-67.7). Corticosteroids reduced the risk of a poor outcome (adjusted hazard ratio [HR] 0.25; 95% CI 0.2-0.4), but this treatment failed in 30.5%. An internally validated nomogram composed of older age, MELD, encephalopathy and ascites at the initiation of corticosteroids accurately predicted the response (C-index 0.82; [95% CI 0.8-0.9]). In responders, MELD significantly improved from days 3 to 14 but remained unchanged in non-responders. MELD on day 7 with a cut-off of 25 (sensitivity 62.5%[95% CI: 47.0-75.8]; specificity 95.2% [95% CI: 89.9-97.8]) was the best univariate predictor of the response. Prolonging corticosteroids did not increase the overall infection risk (adjusted HR 0.75; 95% CI 0.3-2.1).
Conclusion: Older patients with high MELD, encephalopathy or ascites at steroid therapy initiation and during treatment are unlikely to show a favourable response and so prolonged therapy in these patients, especially if they are transplantation candidates, should be avoided.
Corticosteroids in acute‐severe autoimmune hepatits.
© 2022 The Authors. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures

Comment in
-
Editorial: acute severe autoimmune hepatitis-a diagnostic and therapeutic dilemma.Aliment Pharmacol Ther. 2022 Jul;56(1):170-171. doi: 10.1111/apt.16981. Aliment Pharmacol Ther. 2022. PMID: 35689316 No abstract available.
-
Letter: elderly AS-AIH patients need more attention.Aliment Pharmacol Ther. 2023 Jan;57(1):181-182. doi: 10.1111/apt.17240. Epub 2022 Dec 5. Aliment Pharmacol Ther. 2023. PMID: 36468231 No abstract available.
-
Letter: Elderly AS-AIH patients need more attention-Authors' reply.Aliment Pharmacol Ther. 2023 Jan;57(1):183-184. doi: 10.1111/apt.17287. Aliment Pharmacol Ther. 2023. PMID: 36480712 No abstract available.
-
Letter: time to consider Pneumocystis jirovecii pneumonia prophylaxis in treatment of autoimmune hepatitis.Aliment Pharmacol Ther. 2023 May;57(10):1210-1211. doi: 10.1111/apt.17508. Aliment Pharmacol Ther. 2023. PMID: 37094310 No abstract available.
References
-
- Mack CL, Adams D, Assis DN, Kerkar N, Manns MP, Mayo MJ, et al. Diagnosis and Management of Autoimmune Hepatitis in adults and children: 2019 practice guidance and guidelines from the American Association for the Study of Liver Diseases. Hepatology. 2020;72(2):671–722. 10.1002/hep.31065 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
