The tauopathies: Neuroimaging characteristics and emerging experimental therapies
- PMID: 35470528
- PMCID: PMC9545715
- DOI: 10.1111/jon.13001
The tauopathies: Neuroimaging characteristics and emerging experimental therapies
Abstract
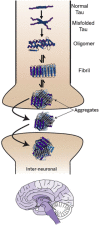
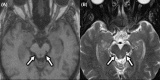
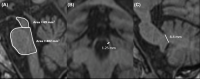
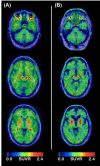
The tauopathies are a heterogeneous group of neurodegenerative disorders in which the prevailing underlying disease process is intracellular deposition of abnormal misfolded tau protein. Diseases often categorized as tauopathies include progressive supranuclear palsy, chronic traumatic encephalopathy, corticobasal degeneration, and frontotemporal lobar degeneration. Tauopathies can be classified through clinical assessment, imaging findings, histologic validation, or molecular biomarkers tied to the underlying disease mechanism. Many tauopathies vary in their clinical presentation and overlap substantially in presentation, making clinical diagnosis of a specific primary tauopathy difficult. Anatomic imaging findings are also rarely specific to a single tauopathy, and when present may not manifest until well after the point at which therapy may be most impactful. Molecular biomarkers hold the most promise for patient care and form a platform upon which emerging diagnostic and therapeutic applications could be developed. One of the most exciting developments utilizing these molecular biomarkers for assessment of tau deposition within the brain is tau-PET imaging utilizing novel ligands that specifically target tau protein. This review will discuss the background, significance, and clinical presentation of each tauopathy with additional attention to the pathologic mechanisms at the protein level. The imaging characteristics will be outlined with select examples of emerging imaging techniques. Finally, current treatment options and emerging therapies will be discussed. This is by no means a comprehensive review of the literature but is instead intended for the practicing radiologist as an overview of a rapidly evolving topic.
Keywords: chronic traumatic encephalopathy; corticobasal degeneration; frontotemporal lobar degeneration; molecular imaging; progressive supranuclear palsy; tau; tauopathies.
© 2022 The Authors. Journal of Neuroimaging published by Wiley Periodicals LLC on behalf of American Society of Neuroimaging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Arendt T, Stieler JT, Holzer M Tau and tauopathies. Brain Res Bull 2016;126:238‐92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

