Metformin in nucleus accumbens core reduces cue-induced cocaine seeking in male and female rats
- PMID: 35470560
- PMCID: PMC9285471
- DOI: 10.1111/adb.13165
Metformin in nucleus accumbens core reduces cue-induced cocaine seeking in male and female rats
Abstract
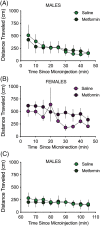
This study investigated the potential therapeutic effects of the FDA-approved drug metformin on cue-induced reinstatement of cocaine seeking. Metformin (dimethyl-biguanide) is a first-line treatment for type II diabetes that, among other mechanisms, is involved in the activation of adenosine monophosphate activated protein kinase (AMPK). Cocaine self-administration and extinction is associated with decreased levels of phosphorylated AMPK within the nucleus accumbens core (NAcore). Previously, it was shown that increasing AMPK activity in the NAcore decreased cue-induced reinstatement of cocaine seeking. Decreasing AMPK activity produced the opposite effect. The goal of the present study was to determine if metformin in the NAcore reduces cue-induced cocaine seeking in adult male and female Sprague Dawley rats. Rats were trained to self-administer cocaine followed by extinction prior to cue-induced reinstatement trials. Metformin microinjected in the NAcore attenuated cue-induced reinstatement in male and female rats. Importantly, metformin's effects on cocaine seeking were not due to a general depression of spontaneous locomotor activity. In female rats, metformin's effects did generalize to a reduction in cue-induced reinstatement of sucrose seeking. These data support a potential role for metformin as a pharmacotherapy for cocaine use disorder but warrant caution given the potential for metformin's effects to generalize to a natural reward in female rats.
Keywords: cocaine; metformin; nucleus accumbens; reinstatement; self-administration.
© 2022 The Authors. Addiction Biology published by John Wiley & Sons Ltd on behalf of Society for the Study of Addiction.
Conflict of interest statement
The authors have no conflicts of interest to report.
Figures




Similar articles
-
Accumbens brain-derived neurotrophic factor (BDNF) transmission inhibits cocaine seeking.Addict Biol. 2019 Sep;24(5):860-873. doi: 10.1111/adb.12638. Epub 2018 Jun 11. Addict Biol. 2019. PMID: 29890020 Free PMC article.
-
Deep brain stimulation of the nucleus accumbens shell attenuates cue-induced reinstatement of both cocaine and sucrose seeking in rats.Behav Brain Res. 2015 Mar 15;281:125-30. doi: 10.1016/j.bbr.2014.12.025. Epub 2014 Dec 18. Behav Brain Res. 2015. PMID: 25529183 Free PMC article.
-
Amperometric measurements of cocaine cue and novel context-evoked glutamate and nitric oxide release in the nucleus accumbens core.J Neurochem. 2020 Jun;153(5):599-616. doi: 10.1111/jnc.14952. Epub 2020 Feb 3. J Neurochem. 2020. PMID: 31901130 Free PMC article.
-
Metformin in Esophageal Carcinoma: Exploring Molecular Mechanisms and Therapeutic Insights.Int J Mol Sci. 2024 Mar 4;25(5):2978. doi: 10.3390/ijms25052978. Int J Mol Sci. 2024. PMID: 38474224 Free PMC article. Review.
-
The anti-inflammatory effect of metformin: The molecular targets.Genes Cells. 2024 Mar;29(3):183-191. doi: 10.1111/gtc.13098. Epub 2024 Feb 4. Genes Cells. 2024. PMID: 38311861 Free PMC article. Review.
Cited by
-
Metformin Prevents Cocaine Sensitization: Involvement of Adenosine Monophosphate-Activated Protein Kinase Trafficking between Subcellular Compartments in the Corticostriatal Reward Circuit.Int J Mol Sci. 2023 Nov 28;24(23):16859. doi: 10.3390/ijms242316859. Int J Mol Sci. 2023. PMID: 38069180 Free PMC article.
-
Effects of metformin on binge-like ethanol drinking and adenosine monophosphate kinase signaling in inbred high drinking in the dark line 1 mice.Alcohol Clin Exp Res (Hoboken). 2024 Dec;48(12):2269-2280. doi: 10.1111/acer.15460. Epub 2024 Nov 26. Alcohol Clin Exp Res (Hoboken). 2024. PMID: 39589266 Free PMC article.
-
Therapeutic effects of metformin on cocaine conditioned place preference and locomotion.Behav Neurosci. 2025 Jun;139(3):122-136. doi: 10.1037/bne0000620. Epub 2025 Feb 27. Behav Neurosci. 2025. PMID: 40014500
-
Repurposing anti-inflammatory medications for alcohol and substance use disorders.Neuropsychopharmacology. 2024 Jan;49(1):317-318. doi: 10.1038/s41386-023-01696-z. Neuropsychopharmacology. 2024. PMID: 37550437 Free PMC article. No abstract available.
References
-
- SAMHSA . Key Substance Use and Mental Health Indicators in the United States: Results from the 2019 National Survey on Drug Use and Health; 2020. https://www.samhsa.gov/data/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

