On-Surface Azide-Alkyne Cycloaddition Reaction: Does It Click with Ruthenium Catalysts?
- PMID: 35470670
- PMCID: PMC9097529
- DOI: 10.1021/acs.langmuir.2c00100
On-Surface Azide-Alkyne Cycloaddition Reaction: Does It Click with Ruthenium Catalysts?
Abstract
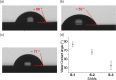
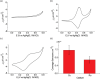
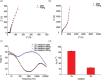
Owing to its simplicity, selectivity, high yield, and the absence of byproducts, the "click" azide-alkyne reaction is widely used in many areas. The reaction is usually catalyzed by copper(I), which selectively produces the 1,4-disubstituted 1,2,3-triazole regioisomer. Ruthenium-based catalysts were later developed to selectively produce the opposite regioselectivity─the 1,5-disubstituted 1,2,3-triazole isomer. Ruthenium-based catalysis, however, remains only tested for click reactions in solution, and the suitability of ruthenium catalysts for surface-based click reactions remains unknown. Also unknown are the electrical properties of the 1,4- and 1,5-regioisomers, and to measure them, both isomers need to be assembled on the electrode surface. Here, we test whether ruthenium catalysts can be used to catalyze surface azide-alkyne reactions to produce 1,5-disubstituted 1,2,3-triazole, and compare their electrochemical properties, in terms of surface coverages and electron transfer kinetics, to those of the compound formed by copper catalysis, 1,4-disubstituted 1,2,3-triazole isomer. Results show that ruthenium(II) complexes catalyze the click reaction on surfaces yielding the 1,5-disubstituted isomer, but the rate of the reaction is remarkably slower than that of the copper-catalyzed reaction, and this is related to the size of the catalyst involved as an intermediate in the reaction. The electron transfer rate constant (ket) for the ruthenium-catalyzed reaction is 30% of that measured for the copper-catalyzed 1,4-isomer. The lower conductivity of the 1,5-isomer is confirmed by performing nonequilibrium Green's function computations on relevant model systems. These findings demonstrate the feasibility of ruthenium-based catalysis of surface click reactions and point toward an electrical method for detecting the isomers of click reactions.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Synthesis of Dense 1,2,3-Triazole Oligomers Consisting Preferentially of 1,5-Disubstituted Units via Ruthenium(II)-Catalyzed Azide-Alkyne Cycloaddition.Polymers (Basel). 2023 May 5;15(9):2199. doi: 10.3390/polym15092199. Polymers (Basel). 2023. PMID: 37177345 Free PMC article.
-
On the Mechanism of Copper(I)-Catalyzed Azide-Alkyne Cycloaddition.Chem Rec. 2016 Jun;16(3):1501-17. doi: 10.1002/tcr.201600002. Epub 2016 May 24. Chem Rec. 2016. PMID: 27216993
-
Computational studies on the regioselectivity of metal-catalyzed synthesis of 1,2,3 triazoles via click reaction: a review.J Mol Model. 2015 Oct;21(10):264. doi: 10.1007/s00894-015-2810-2. Epub 2015 Sep 18. J Mol Model. 2015. Retraction in: J Mol Model. 2019 Mar 7;25(4):86. doi: 10.1007/s00894-019-3978-7. PMID: 26385849 Retracted. Review.
-
Visible-Light-Mediated Click Chemistry for Highly Regioselective Azide-Alkyne Cycloaddition by a Photoredox Electron-Transfer Strategy.Chemistry. 2020 May 4;26(25):5694-5700. doi: 10.1002/chem.202000252. Epub 2020 Apr 17. Chemistry. 2020. PMID: 31953964
-
Click to join peptides/proteins together.Chem Asian J. 2011 Oct 4;6(10):2606-16. doi: 10.1002/asia.201100329. Chem Asian J. 2011. PMID: 22043498 Review.
Cited by
-
Electrostatic catalysis of a click reaction in a microfluidic cell.Nat Commun. 2024 Jan 26;15(1):790. doi: 10.1038/s41467-024-44716-2. Nat Commun. 2024. PMID: 38278792 Free PMC article.
-
Mutually Orthogonal Bioorthogonal Reactions: Selective Chemistries for Labeling Multiple Biomolecules Simultaneously.Top Curr Chem (Cham). 2024 Jul 6;382(3):24. doi: 10.1007/s41061-024-00467-8. Top Curr Chem (Cham). 2024. PMID: 38971884 Free PMC article. Review.
-
Tunable Nanoscale Metal‒Molecule‒Semiconductor Junctions via Light-Controlled Molecular Orientation.Small. 2025 Jul;21(29):e2412438. doi: 10.1002/smll.202412438. Epub 2025 May 19. Small. 2025. PMID: 40384288 Free PMC article.
References
-
- Katritzky A. R.; Rees C. W.; Scriven E. F.. Comprehensive Heterocyclic Chemistry II; Pergamon, 1996; pp 1–11628.
-
- Yamaguchi R.; Hosomi T.; Otani M.; Nagashima K.; Takahashi T.; Zhang G.; Kanai M.; Masai H.; Terao J.; Yanagida T. Maximizing Conversion of Surface Click Reactions for Versatile Molecular Modification on Metal Oxide Nanowires. Langmuir 2021, 37, 5172–5179. 10.1021/acs.langmuir.1c00106. - DOI - PubMed
-
- Lattimer J. R. C.; Brunschwig B. S.; Lewis N. S.; Gray H. B. Redox Properties of Mixed Methyl/Vinylferrocenyl Monolayers on Si (111) Surfaces. J. Phys. Chem. C 2013, 117, 27012–27022. 10.1021/jp409958c. - DOI
LinkOut - more resources
Full Text Sources

