Expert Recommendations for Using Time-in-Range and Other Continuous Glucose Monitoring Metrics to Achieve Patient-Centered Glycemic Control in People With Diabetes
- PMID: 35470692
- PMCID: PMC10563535
- DOI: 10.1177/19322968221088601
Expert Recommendations for Using Time-in-Range and Other Continuous Glucose Monitoring Metrics to Achieve Patient-Centered Glycemic Control in People With Diabetes
Abstract
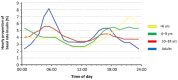
New metrics for assessing glycemic control beyond HbA1c have recently emerged due to the increasing use of continuous glucose monitoring (CGM) in diabetes clinical practice. Among them, time in range (TIR) has appeared as a simple and intuitive metric that correlates inversely with HbA1c and has also been newly linked to the risk of long-term diabetes complications. The International Consensus on Time in Range established a series of target glucose ranges (TIR, time below range and time above range) and recommendations for time spent within these ranges for different diabetes populations. These parameters should be evaluated together with the ambulatory glucose profile (AGP). Using standardized visual reporting may help people with diabetes and healthcare professionals in the evaluation of glucose control in frequent clinical situations. The objective of the present review is to provide practical insights to quick interpretation of patient-centered metrics based on flash glucose monitoring data, as well as showing some visual examples of common clinical situations and giving practical recommendations for their management.
Keywords: ambulatory glucose profile; continuous glucose monitoring; flash glucose monitoring; glycemic variability; hypoglycemia; time in range.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: VB has received speaker/advisory honoraria from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Esteve, Janssen, Merck, Mundi- pharma, Novartis, Novo Nordisk, Roche, Sanofi. EAH has received honoraria from Abbott, Novo Nordisk, Sanofi, Lilly and Roche Diabetes Care. RCH has received speaker honoraria from Abbott, Lilly, Medtronic, Novo-Nordisk and Sanofi-Aventis and has consulted for Abbott, Lilly, Novo-Nordisk and Sanofi. GDS has received advisory honoraria/research support from NovoNordisk, Lilly, Medtronic and Abbott. NGPV has received speaker honorary from Sanofi, Novo Nordisk, Boehringer Ingelheim, Lilly Astra Zeneca, Abbott, and MSD. MJPC have received fees for collaborations from Abbott Diabetes Care, Ascensia Diabetes Care, Novo Nordisk and Sanofi. FJAB has received consultant/advisor honoraria from Abbott Diabetes Care, AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, LifeScan, MannKind Co., Medtronic, Menarini, Merck, Novartis, Novo Nordisk, Sanofi; Speaker honoraria from Abbott Diabetes Care, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, LifeScan, Eli Lilly, Madaus, Medtronic, Menarini, Merck, Novartis, Novo Nordisk, Sanofi; Grant support from Novo Nordisk, Sanofi.
Figures


References
-
- Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, et al.. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. - PubMed
-
- Campbell L, Pepper T, Shipman K. HbA1c: a review of non-glycaemic variables. J Clin Pathol. 2019;72:12-19. - PubMed
-
- Bellido V, Pinés-Corrales PJ, Villar-Taibo R, Ampudia-Blasco FJ. Time-in-range for monitoring glucose control: Is it time for a change? Diabetes Res Clin Pract. 2021;177:108917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

