Quantitative MRI Characterization of the Extremely Preterm Brain at Adolescence: Atypical versus Neurotypical Developmental Pathways
- PMID: 35471112
- PMCID: PMC9340244
- DOI: 10.1148/radiol.210385
Quantitative MRI Characterization of the Extremely Preterm Brain at Adolescence: Atypical versus Neurotypical Developmental Pathways
Abstract
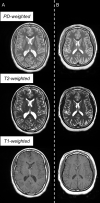
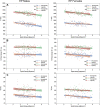
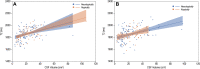
Background Extremely preterm (EP) birth is associated with higher risks of perinatal white matter (WM) injury, potentially causing abnormal neurologic and neurocognitive outcomes. MRI biomarkers distinguishing individuals with and without neurologic disorder guide research on EP birth antecedents, clinical correlates, and prognoses. Purpose To compare multiparametric quantitative MRI (qMRI) parameters of EP-born adolescents with autism spectrum disorder, cerebral palsy, epilepsy, or cognitive impairment (ie, atypically developing) with those without (ie, neurotypically developing), characterizing sex-stratified brain development. Materials and Methods This prospective multicenter study included individuals aged 14-16 years born EP (Extremely Low Gestational Age Newborns-Environmental Influences on Child Health Outcomes Study, or ELGAN-ECHO). Participants underwent 3.0-T MRI evaluation from 2017 to 2019. qMRI outcomes were compared for atypically versus neurotypically developing adolescents and for girls versus boys. Sex-stratified multiple regression models were used to examine associations between spatial entropy density (SEd) and T1, T2, and cerebrospinal fluid (CSF)-normalized proton density (nPD), and between CSF volume and T2. Interaction terms modeled differences in slopes between atypically versus neurotypically developing adolescents. Results A total of 368 adolescents were classified as 116 atypically (66 boys) and 252 neurotypically developing (125 boys) participants. Atypically versus neurotypically developing girls had lower nPD (mean, 557 10 × percent unit [pu] ± 46 [SD] vs 573 10 × pu ± 43; P = .04), while atypically versus neurotypically developing boys had longer T1 (814 msec ± 57 vs 789 msec ± 82; P = .01). Atypically developing girls versus boys had lower nPD and shorter T2 (eg, in WM, 557 10 × pu ± 46 vs 580 10 × pu ± 39 for nPD [P = .006] and 86 msec ± 3 vs 88 msec ± 4 for T2 [P = .003]). Atypically versus neurotypically developing boys had a more moderate negative association between T1 and SEd (slope, -32.0 msec per kB/cm3 [95% CI: -49.8, -14.2] vs -62.3 msec per kB/cm3 [95% CI: -79.7, -45.0]; P = .03). Conclusion Atypically developing participants showed sexual dimorphisms in the cerebrospinal fluid-normalized proton density (nPD) and T2 of both white matter (WM) and gray matter. Atypically versus neurotypically developing girls had lower WM nPD, while atypically versus neurotypically developing boys had longer WM T1 and more moderate T1 associations with microstructural organization in WM. © RSNA, 2022 Online supplemental material is available for this article.
Conflict of interest statement
Figures

References
-
- Volpe JJ . Dysmaturation of premature brain: importance, cellular mechanisms, and potential interventions . Pediatr Neurol 2019. ; 95 : 42– 66 . - PubMed
-
- Lebel C , Treit S , Beaulieu C . A review of diffusion MRI of typical white matter development from early childhood to young adulthood . NMR Biomed 2019. ; 32 ( 4 ): e3778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

