Molecular Dynamics and Theratyping in Airway and Gut Organoids Reveal R352Q-CFTR Conductance Defect
- PMID: 35471184
- PMCID: PMC9273222
- DOI: 10.1165/rcmb.2021-0337OC
Molecular Dynamics and Theratyping in Airway and Gut Organoids Reveal R352Q-CFTR Conductance Defect
Abstract
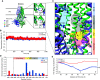
A significant challenge to making targeted cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapies accessible to all individuals with cystic fibrosis (CF) are many mutations in the CFTR gene that can cause CF, most of which remain uncharacterized. Here, we characterized the structural and functional defects of the rare CFTR mutation R352Q, with a potential role contributing to intrapore chloride ion permeation, in patient-derived cell models of the airway and gut. CFTR function in differentiated nasal epithelial cultures and matched intestinal organoids was assessed using an ion transport assay and forskolin-induced swelling assay, respectively. CFTR potentiators (VX-770, GLPG1837, and VX-445) and correctors (VX-809, VX-445, with or without VX-661) were tested. Data from R352Q-CFTR were compared with data of 20 participants with mutations with known impact on CFTR function. R352Q-CFTR has residual CFTR function that was restored to functional CFTR activity by CFTR potentiators but not the corrector. Molecular dynamics simulations of R352Q-CFTR were carried out, which indicated the presence of a chloride conductance defect, with little evidence supporting a gating defect. The combination approach of in vitro patient-derived cell models and in silico molecular dynamics simulations to characterize rare CFTR mutations can improve the specificity and sensitivity of modulator response predictions and aid in their translational use for CF precision medicine.
Keywords: intestinal organoid; molecular dynamics simulations; nasal epithelial cells; personalized medicine; rare cystic fibrosis.
Figures




References
-
- Ruseckaite R, Ahern S, Ranger T, Dean J, Gardam M, Bell S, et al. Australian Cystic Fibrosis Data Registry . The Australian Cystic Fibrosis Data Registry Annual Report 2017. Melbourne, Australia: Monash University, Department of Epidemiology and Preventive Medicine; March 2019. Report No. 20 [accessed 2021 Jun 30]. Available from: https://www.cysticfibrosis.org.au/getmedia/24e94d66-29fa-4e3f-8e65-21ee2...
-
- Marson FAL, Bertuzzo CS, Ribeiro JD. Classification of CFTR mutation classes. Lancet Respir Med . 2016;4:e37–e38. - PubMed
-
- Loo TW, Bartlett MC, Clarke DM. Corrector VX-809 stabilizes the first transmembrane domain of CFTR. Biochem Pharmacol . 2013;86:612–619. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

