Neutrophil extracellular traps arm DC vaccination against NPM-mutant myeloproliferation
- PMID: 35471185
- PMCID: PMC9068207
- DOI: 10.7554/eLife.69257
Neutrophil extracellular traps arm DC vaccination against NPM-mutant myeloproliferation
Abstract
Neutrophil extracellular traps (NETs) are web-like chromatin structures composed by dsDNA and histones, decorated with antimicrobial proteins. Their interaction with dendritic cells (DCs) allows DC activation and maturation toward presentation of NET-associated antigens. Differently from other types of cell death that imply protein denaturation, NETosis preserves the proteins localized onto the DNA threads for proper enzymatic activity and conformational status, including immunogenic epitopes. Besides neutrophils, leukemic cells can release extracellular traps displaying leukemia-associated antigens, prototypically mutant nucleophosmin (NPMc+) that upon mutation translocates from nucleolus to the cytoplasm localizing onto NET threads. We tested NPMc+ immunogenicity through a NET/DC vaccine to treat NPMc-driven myeloproliferation in transgenic and transplantable models. Vaccination with DC loaded with NPMc+ NET (NPMc+ NET/DC) reduced myeloproliferation in transgenic mice, favoring the development of antibodies to mutant NPMc and the induction of a CD8+ T-cell response. The efficacy of this vaccine was also tested in mixed NPMc/WT bone marrow (BM) chimeras in a competitive BM transplantation setting, where the NPMc+ NET/DC vaccination impaired the expansion of NPMc+ in favor of WT myeloid compartment. NPMc+ NET/DC vaccination also achieved control of an aggressive leukemia transduced with mutant NPMc, effectively inducing an antileukemia CD8 T-cell memory response.
Keywords: extracellular traps; immunology; inflammation; mouse; myeloproliferation; nucleophosmin; vaccine.
© 2022, Tripodo et al.
Conflict of interest statement
CT, BB, EJ, VC, CC, PP, LB, CV, MP, MP, PC, ML, PV, AC, MC, SS No competing interests declared
Figures

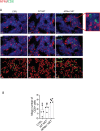
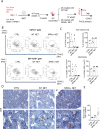
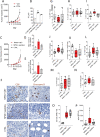

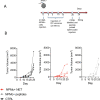
References
-
- Arranz L, Sánchez-Aguilera A, Martín-Pérez D, Isern J, Langa X, Tzankov A, Lundberg P, Muntión S, Tzeng YS, Lai DM, Schwaller J, Skoda RC, Méndez-Ferrer S. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature. 2014;512:78–81. doi: 10.1038/nature13383. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

