Placental dysfunction influences fetal monocyte subpopulation gene expression in preterm birth
- PMID: 35471950
- PMCID: PMC9220934
- DOI: 10.1172/jci.insight.155482
Placental dysfunction influences fetal monocyte subpopulation gene expression in preterm birth
Abstract
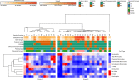
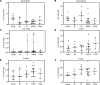
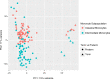
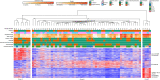
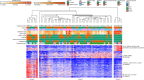
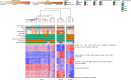
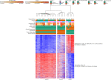
The placenta is the primary organ for immune regulation, nutrient delivery, gas exchange, protection against environmental toxins, and physiologic perturbations during pregnancy. Placental inflammation and vascular dysfunction during pregnancy are associated with a growing list of prematurity-related complications. The goal of this study was to identify differences in gene expression profiles in fetal monocytes - cells that persist and differentiate postnatally - according to distinct placental histologic domains. Here, by using bulk RNA-Seq, we report that placental lesions are associated with gene expression changes in fetal monocyte subsets. Specifically, we found that fetal monocytes exposed to acute placental inflammation upregulate biological processes related to monocyte activation, monocyte chemotaxis, and platelet function, while monocytes exposed to maternal vascular malperfusion lesions downregulate these processes. Additionally, we show that intermediate monocytes might be a source of mitogens, such as HBEGF, NRG1, and VEGFA, implicated in different outcomes related to prematurity. This is the first study to our knowledge to show that placental lesions are associated with unique changes in fetal monocytes and monocyte subsets. As fetal monocytes persist and differentiate into various phagocytic cells following birth, our study may provide insight into morbidity related to prematurity and ultimately potential therapeutic targets.
Keywords: Adaptive immunity; Chemokines; Monocytes; Reproductive Biology; Vascular Biology.
Figures









References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

