Incidence of anogenital warts after the introduction of the quadrivalent HPV vaccine program in Manitoba, Canada
- PMID: 35472093
- PMCID: PMC9041799
- DOI: 10.1371/journal.pone.0267646
Incidence of anogenital warts after the introduction of the quadrivalent HPV vaccine program in Manitoba, Canada
Abstract
Background: The incidence of anogenital warts (AGW) decreased after the introduction of the quadrivalent human papillomavirus (qHPV) vaccine in multiple jurisdictions. We studied how comparing AGW incidence rates with different outcomes affects the interpretation of the qHPV vaccination program. To do this, we replicated multiple study designs within a single jurisdiction (Manitoba).
Methods: We measured the incidence rates of AGW, AGW-related prescriptions, chlamydia, and gonorrhea (the latter two as sham outcomes) between 2001 and 2017 using several clinical and administrative health databases from Manitoba. We then used incidence rate ratios (IRRs) to compare, for each outcome, the rate for the 1997-1998 birth cohort (the first cohorts eligible for the publicly funded qHPV vaccination program) and the older 1995-1996 birth cohort.
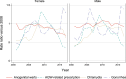
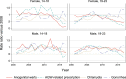
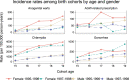
Results: AGW incidence in Manitoba dropped 72% (95% confidence interval 54-83%) among 16-18 year-old girls and 51% (14-72%) among boys after the introduction of the female-only qHPV vaccination program. Trends in AGW-related prescriptions were different from trends in AGW diagnoses as these prescriptions peaked shortly after the introduction of the publicly funded qHPV vaccine program. Chlamydia and gonorrhea incidence rates also decreased 12% (5-18%) and 16% (-1-30%), respectively, for 16-18 year-old girls.
Conclusions: The publicly funded school-based qHPV vaccine program reduced AGW incidence in Manitoba by three-quarters in young females. AGW-related prescriptions are a poor proxy for medically attended AGW after the introduction of the publicly funded qHPV vaccination program. Different sexual habits in adolescents are, at most, responsible for a small portion of the reduction in AGW incidence.
Conflict of interest statement
CHR has received an unrestricted research grant from Pfizer for an unrelated study. KW does not have a financial relationship to disclose. EK has received consulting fees from Merck Canada and GlaxoSmithKline for unrelated studies. EK has received honoraria and travel expenses from Merck Canada. SMM received research funding from Assurex, GSK, Merck, Pfizer, Roche and Sanofi for unrelated studies and is/was a member of advisory boards for GSK, Merck, Sanofi and Seqirus. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Garland SM, Steben M, Sings HL, James M, Lu S, Railkar R, et al. Natural History of Genital Warts: Analysis of the Placebo Arm of 2 Randomized Phase III Trials of a Quadrivalent Human Papillomavirus (Types 6, 11, 16, and 18) Vaccine. The Journal of infectious diseases. 2009;199(6):805–14. doi: 10.1086/597071 - DOI - PubMed
-
- Drolet M, Bénard É, Boily M-C, Ali H, Baandrup L, Bauer H, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. The Lancet Infectious Diseases. 2015;15(5):565–80. doi: 10.1016/S1473-3099(14)71073-4 - DOI - PMC - PubMed
-
- Drolet M, Bénard É, Pérez N, Brisson M, Ali H, Boily M-C, et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. The Lancet. 2019;394(10197):497–509. doi: 10.1016/S0140-6736(19)30298-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

