COVID-19 active case findings based on self-collected saliva samples with CRISPR-Cas12a detection
- PMID: 35473361
- PMCID: PMC9379603
- DOI: 10.1177/15353702221090181
COVID-19 active case findings based on self-collected saliva samples with CRISPR-Cas12a detection
Abstract
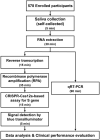
COVID-19 is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus affecting the world population. Early detection has become one of the most successful strategies to alleviate the epidemic and pandemic of this contagious coronavirus. Surveillance testing programs have been initiated in many countries worldwide to prevent the outbreak of COVID-19. In this study, we demonstrated that our previously established clustered regularly interspaced short palindromic repeats (CRISPR)-Cas12a-based assay could detect variants of concern during 2021 in Thailand, including Alpha, Beta, and Delta strains as well as Omicron strain in early 2022. In combination with the newly designed saliva collection funnel, we established a safe, simple, economical, and efficient self-collection protocol for the COVID-19 screening process. We successfully utilized the assay in an active case finding with a total number of 578 asymptomatic participants to detect the SARS-CoV-2 in saliva samples. We finally demonstrated that the validation and evaluation in a large-scale setting could provide valuable information and elaborate the practicality of the test in real-world settings. Our optimized protocol yielded effective results with high sensitivity, specificity, and diagnostic accuracy (96.86%). In addition, this study demonstrates COVID-19 active case findings in low-resource settings, which would be feasible and attractive for surveillance and outbreak prevention in the future.
Keywords: COVID-19; CRISPR-Cas12a; RPA; active case findings; qRT-PCR; saliva samples.
Conflict of interest statement
Figures




Similar articles
-
Detection of Major SARS-CoV-2 Variants of Concern in Clinical Samples via CRISPR-Cas12a-Mediated Mutation-Specific Assay.ACS Synth Biol. 2022 May 20;11(5):1811-1823. doi: 10.1021/acssynbio.1c00643. Epub 2022 Apr 28. ACS Synth Biol. 2022. PMID: 35481381
-
Effectiveness and cost-effectiveness of four different strategies for SARS-CoV-2 surveillance in the general population (CoV-Surv Study): a structured summary of a study protocol for a cluster-randomised, two-factorial controlled trial.Trials. 2021 Jan 8;22(1):39. doi: 10.1186/s13063-020-04982-z. Trials. 2021. PMID: 33419461 Free PMC article.
-
Detection of severe acute respiratory syndrome coronavirus 2 and influenza viruses based on CRISPR-Cas12a.Exp Biol Med (Maywood). 2021 Feb;246(4):400-405. doi: 10.1177/1535370220963793. Epub 2020 Nov 5. Exp Biol Med (Maywood). 2021. PMID: 33153299 Free PMC article.
-
Potential of CRISPR/Cas system in the diagnosis of COVID-19 infection.Expert Rev Mol Diagn. 2021 Nov;21(11):1179-1189. doi: 10.1080/14737159.2021.1970535. Epub 2021 Nov 9. Expert Rev Mol Diagn. 2021. PMID: 34409907 Free PMC article. Review.
-
Diagnostic efficiency of RPA/RAA integrated CRISPR-Cas technique for COVID-19: A systematic review and meta-analysis.PLoS One. 2022 Oct 26;17(10):e0276728. doi: 10.1371/journal.pone.0276728. eCollection 2022. PLoS One. 2022. PMID: 36288366 Free PMC article.
Cited by
-
Classification of salivary bacteriome in asymptomatic COVID-19 cases based on long-read nanopore sequencing.Exp Biol Med (Maywood). 2022 Nov;247(21):1937-1946. doi: 10.1177/15353702221118091. Epub 2022 Sep 8. Exp Biol Med (Maywood). 2022. PMID: 36082397 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

