Increased LCN2 (lipocalin 2) in the RPE decreases autophagy and activates inflammasome-ferroptosis processes in a mouse model of dry AMD
- PMID: 35473441
- PMCID: PMC9809950
- DOI: 10.1080/15548627.2022.2062887
Increased LCN2 (lipocalin 2) in the RPE decreases autophagy and activates inflammasome-ferroptosis processes in a mouse model of dry AMD
Abstract
In dry age-related macular degeneration (AMD), LCN2 (lipocalin 2) is upregulated. Whereas LCN2 has been implicated in AMD pathogenesis, the mechanism remains unknown. Here, we report that in retinal pigmented epithelial (RPE) cells, LCN2 regulates macroautophagy/autophagy, in addition to maintaining iron homeostasis. LCN2 binds to ATG4B to form an LCN2-ATG4B-LC3-II complex, thereby regulating ATG4B activity and LC3-II lipidation. Thus, increased LCN2 reduced autophagy flux. Moreover, RPE cells from cryba1 KO, as well as sting1 KO and Sting1Gt mutant mice (models with abnormal iron chelation), showed decreased autophagy flux and increased LCN2, indicative of CGAS- and STING1-mediated inflammasome activation. Live cell imaging of RPE cells with elevated LCN2 also showed a correlation between inflammasome activation and increased fluorescence intensity of the Liperfluo dye, indicative of oxidative stress-induced ferroptosis. Interestingly, both in human AMD patients and in mouse models with a dry AMD-like phenotype (cryba1 cKO and KO), the LCN2 homodimer variant is increased significantly compared to the monomer. Sub-retinal injection of the LCN2 homodimer secreted by RPE cells into NOD-SCID mice leads to retinal degeneration. In addition, we generated an LCN2 monoclonal antibody that neutralizes both the monomer and homodimer variants and rescued autophagy and ferroptosis activities in cryba1 cKO mice. Furthermore, the antibody rescued retinal function in cryba1 cKO mice as assessed by electroretinography. Here, we identify a molecular pathway whereby increased LCN2 elicits pathophysiology in the RPE, cells known to drive dry AMD pathology, thus providing a possible therapeutic strategy for a disease with no current treatment options.Abbreviations: ACTB: actin, beta; Ad-GFP: adenovirus-green fluorescent protein; Ad-LCN2: adenovirus-lipocalin 2; Ad-LCN2-GFP: adenovirus-LCN2-green fluorescent protein; LCN2AKT2: AKT serine/threonine kinase 2; AMBRA1: autophagy and beclin 1 regulator 1; AMD: age-related macular degeneration; ARPE19: adult retinal pigment epithelial cell line-19; Asp278: aspartate 278; ATG4B: autophagy related 4B cysteine peptidase; ATG4C: autophagy related 4C cysteine peptidase; ATG7: autophagy related 7; ATG9B: autophagy related 9B; BLOC-1: biogenesis of lysosomal organelles complex 1; BLOC1S1: biogenesis of lysosomal organelles complex 1 subunit 1; C57BL/6J: C57 black 6J; CGAS: cyclic GMP-AMP synthase; ChQ: chloroquine; cKO: conditional knockout; Cys74: cysteine 74; Dab2: DAB adaptor protein 2; Def: deferoxamine; DHE: dihydroethidium; DMSO: dimethyl sulfoxide; ERG: electroretinography; FAC: ferric ammonium citrate; Fe2+: ferrous; FTH1: ferritin heavy chain 1; GPX: glutathione peroxidase; GST: glutathione S-transferase; H2O2: hydrogen peroxide; His280: histidine 280; IFNL/IFNλ: interferon lambda; IL1B/IL-1β: interleukin 1 beta; IS: Inner segment; ITGB1/integrin β1: integrin subunit beta 1; KO: knockout; LC3-GST: microtubule associated protein 1 light chain 3-GST; C-terminal fusion; MAP1LC3/LC3: microtubule associated protein 1 light chain 3; LCN2: lipocalin 2; mAb: monoclonal antibody; MDA: malondialdehyde; MMP9: matrix metallopeptidase 9; NLRP3: NLR family pyrin domain containing 3; NOD-SCID: nonobese diabetic-severe combined immunodeficiency; OS: outer segment; PBS: phosphate-buffered saline; PMEL/PMEL17: premelanosome protein; RFP: red fluorescent protein; rLCN2: recombinant LCN2; ROS: reactive oxygen species; RPE SM: retinal pigmented epithelium spent medium; RPE: retinal pigment epithelium; RSL3: RAS-selective lethal; scRNAseq: single-cell ribonucleic acid sequencing; SD-OCT: spectral domain optical coherence tomography; shRNA: small hairpin ribonucleic acid; SM: spent medium; SOD1: superoxide dismutase 1; SQSTM1/p62: sequestosome 1; STAT1: signal transducer and activator of transcription 1; STING1: stimulator of interferon response cGAMP interactor 1; TYR: tyrosinase; VCL: vinculin; WT: wild type.
Keywords: ATG4B; autophagy; dry age-related macular degeneration; ferroptosis; inflammasome; iron; lipocalin 2; monoclonal/neutralizing antibody; oxidative stress; retinal pigmented epithelial cells.
Conflict of interest statement
SG, SH, DS and NS are inventors in a US patent filed by the University of Pittsburgh. The remaining authors declare no competing interests.
Figures
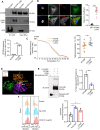
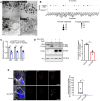
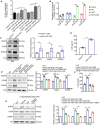
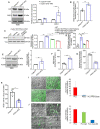

References
-
- Jaberi SA, Cohen A, D’Souza C, et al. Lipocalin-2: structure, function, distribution and role in metabolic disorders. Biomed Pharmacother. 2021. Oct;142:112002. PMID: 34463264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
