Evaluation of electropolymerized molecularly imprinted polymers (E-MIPs) on disposable electrodes for detection of SARS-CoV-2 in saliva
- PMID: 35473858
- PMCID: PMC8974637
- DOI: 10.1016/j.aca.2022.339777
Evaluation of electropolymerized molecularly imprinted polymers (E-MIPs) on disposable electrodes for detection of SARS-CoV-2 in saliva
Abstract
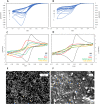
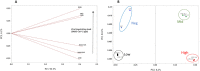
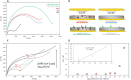
We investigate electropolymerized molecularly imprinted polymers (E-MIPs) for the selective recognition of SARS-CoV-2 whole virus. E-MIPs imprinted with SARS-CoV-2 pseudoparticles (pps) were electrochemically deposited onto screen printed electrodes by reductive electropolymerization, using the water-soluble N-hydroxmethylacrylamide (NHMA) as functional monomer and crosslinked with N,N'-methylenebisacrylamide (MBAm). E-MIPs for SARS-CoV-2 showed selectivity for template SARS-CoV-2 pps, with an imprinting factor of 3:1, and specificity (significance = 0.06) when cross-reacted with other respiratory viruses. E-MIPs detected the presence of SARS-CoV-2 pps in <10 min with a limit of detection of 4.9 log10 pfu/mL, suggesting their suitability for detection of SARS-CoV-2 with minimal sample preparation. Using electrochemical impedance spectroscopy (EIS) and principal component analysis (PCA), the capture of SARS-CoV-2 from real patient saliva samples was also evaluated. Fifteen confirmed COVID-19 positive and nine COVID-19 negative saliva samples were compared against the established loop-mediated isothermal nucleic acid amplification (LAMP) technique used by the UK National Health Service. EIS data demonstrated a PCA discrimination between positive and negative LAMP samples. A threshold real impedance signal (ZRe) ≫ 4000 Ω and a corresponding charge transfer resistance (RCT) ≫ 6000 Ω was indicative of absence of virus (COVID-19 negative) in agreement with values obtained for our control non-imprinted polymer control. A ZRe at or below a threshold value of 600 Ω with a corresponding RCT of <1200 Ω was indicative of a COVID-19 positive sample. The presence of virus was confirmed by treatment of E-MIPs with a SARS-CoV-2 specific monoclonal antibody.
Keywords: Biosensor; COVID-19; Electrochemical impedance spectroscopy; Electrochemical polymerization; Molecularly imprinted polymers; SARS-CoV-2.
Copyright © 2022 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Devi A.N. Overview of COVID-19 pandemic: transmission, epidemiology and diagnosis. Journal of Pharmaceutical Research International. 2021;33:174–181.
-
- Dey P., Vaijayanthimala S., Dalvi V.S., Jain A., Gola D., Bajpai M., Bharti R.K., Chauhan N. COVID-19: understanding the pandemic emergence, impact and infection prevalence worldwide. J. Pure Appl. Microbiol. 2020;14:2235–2251.
MeSH terms
Substances
Grants and funding
- BBS/E/I/00007034/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007038/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/00007039/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/I/COV07001/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

