Heterogeneity of Acetylcholine Receptor Autoantibody-Mediated Complement Activity in Patients With Myasthenia Gravis
- PMID: 35473886
- PMCID: PMC9128035
- DOI: 10.1212/NXI.0000000000001169
Heterogeneity of Acetylcholine Receptor Autoantibody-Mediated Complement Activity in Patients With Myasthenia Gravis
Erratum in
-
Heterogeneity of Acetylcholine Receptor Autoantibody-Mediated Complement Activity in Patients With Myasthenia Gravis.Neurol Neuroimmunol Neuroinflamm. 2022 Aug 3;9(5):e200017. doi: 10.1212/NXI.0000000000200017. Print 2022 Sep. Neurol Neuroimmunol Neuroinflamm. 2022. PMID: 35922151 Free PMC article. No abstract available.
Abstract
Background and objectives: Autoantibodies targeting the acetylcholine receptor (AChR), found in patients with myasthenia gravis (MG), mediate pathology through 3 mechanisms: complement-directed tissue damage, blocking of the acetylcholine binding site, and internalization of the AChR. Clinical assays, used to diagnose and monitor patients, measure only autoantibody binding. Consequently, they are limited in providing association with disease burden, understanding of mechanistic heterogeneity, and monitoring therapeutic response. The objective of this study was to develop a cell-based assay that measures AChR autoantibody-mediated complement membrane attack complex (MAC) formation.
Methods: An HEK293T cell line-modified using CRISPR/Cas9 genome editing to disrupt expression of the complement regulator genes (CD46, CD55, and CD59)-was used to measure AChR autoantibody-mediated MAC formation through flow cytometry.
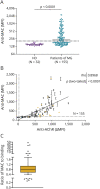
Results: Serum samples (n = 155) from 96 clinically confirmed AChR MG patients, representing a wide range of disease burden and autoantibody titer, were tested along with 32 healthy donor (HD) samples. AChR autoantibodies were detected in 139 of the 155 (89.7%) MG samples through a cell-based assay. Of the 139 AChR-positive samples, autoantibody-mediated MAC formation was detected in 83 (59.7%), whereas MAC formation was undetectable in the HD group or AChR-positive samples with low autoantibody levels. MAC formation was positively associated with autoantibody binding in most patient samples; ratios (mean fluorescence intensity) of MAC formation to AChR autoantibody binding ranged between 0.27 and 48, with a median of 0.79 and an interquartile range of 0.43 (0.58-1.1). However, the distribution of ratios was asymmetric and included extreme values; 16 samples were beyond the 10-90 percentile, with high MAC to low AChR autoantibody binding ratio or the reverse. Correlation between MAC formation and clinical disease scores suggested a modest positive association (rho = 0.34, p = 0.0023), which included a subset of outliers that did not follow this pattern. MAC formation did not associate with exposure to immunotherapy, thymectomy, or MG subtypes defined by age-of-onset.
Discussion: A novel assay for evaluating AChR autoantibody-mediated complement activity was developed. A subset of patients that lacks association between MAC formation and autoantibody binding or disease burden was identified. The assay may provide a better understanding of the heterogeneous autoantibody molecular pathology and identify patients expected to benefit from complement inhibitor therapy.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures



References
-
- Vincent A. Unravelling the pathogenesis of myasthenia gravis. Nat Rev Immunol. 2002;2(10):797-804. - PubMed
-
- Gilhus NE, Skeie GO, Romi F, Lazaridis K, Zisimopoulou P, Tzartos S. Myasthenia gravis–autoantibody characteristics and their implications for therapy. Nat Rev Neurol. 2016;12(5):259-268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
