Loss of perivascular aquaporin-4 localization impairs glymphatic exchange and promotes amyloid β plaque formation in mice
- PMID: 35473943
- PMCID: PMC9040291
- DOI: 10.1186/s13195-022-00999-5
Loss of perivascular aquaporin-4 localization impairs glymphatic exchange and promotes amyloid β plaque formation in mice
Abstract
Background: Slowed clearance of amyloid β (Aβ) is believed to underlie the development of Aβ plaques that characterize Alzheimer's disease (AD). Aβ is cleared in part by the glymphatic system, a brain-wide network of perivascular pathways that supports the exchange of cerebrospinal and brain interstitial fluid. Glymphatic clearance, or perivascular CSF-interstitial fluid exchange, is dependent on the astroglial water channel aquaporin-4 (AQP4) as deletion of Aqp4 in mice slows perivascular exchange, impairs Aβ clearance, and promotes Aβ plaque formation.
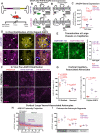
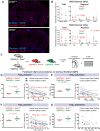
Methods: To define the role of AQP4 in human AD, we evaluated AQP4 expression and localization in a human post mortem case series. We then used the α-syntrophin (Snta1) knockout mouse model which lacks perivascular AQP4 localization to evaluate the effect that loss of perivascular AQP4 localization has on glymphatic CSF tracer distribution. Lastly, we crossed this line into a mouse model of amyloidosis (Tg2576 mice) to evaluate the effect of AQP4 localization on amyloid β levels.
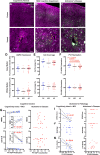
Results: In the post mortem case series, we observed that the perivascular localization of AQP4 is reduced in frontal cortical gray matter of subjects with AD compared to cognitively intact subjects. This decline in perivascular AQP4 localization was associated with increasing Aβ and neurofibrillary pathological burden, and with cognitive decline prior to dementia onset. In rodent studies, Snta1 gene deletion slowed CSF tracer influx and interstitial tracer efflux from the mouse brain and increased amyloid β levels.
Conclusions: These findings suggest that the loss of perivascular AQP4 localization may contribute to the development of AD pathology in human populations.
Keywords: AQP4; Alzheimer’s disease; Amyloid β; Aquaporin-4; Astrocyte; Perivascular; glymphatic; α-Syntrophin.
© 2022. The Author(s).
Conflict of interest statement
The neuropathological case series included in this study was funded through a Sponsored Collaborative Agreement with GlaxoSmithKline to JJI. The other authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

