Targeting NAD+: is it a common strategy to delay heart aging?
- PMID: 35474295
- PMCID: PMC9042931
- DOI: 10.1038/s41420-022-01031-3
Targeting NAD+: is it a common strategy to delay heart aging?
Abstract
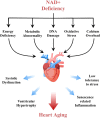
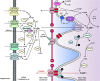
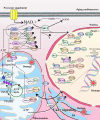
Heart aging is the main susceptible factor to coronary heart disease and significantly increases the risk of heart failure, especially when the aging heart is suffering from ischemia-reperfusion injury. Numerous studies with NAD+ supplementations have suggested its use in anti-aging treatment. However, systematic reviews regarding the overall role of NAD+ in cardiac aging are scarce. The relationship between NAD+ signaling and heart aging has yet to be clarified. This review comprehensively summarizes the current studies on the role of NAD+ signaling in delaying heart aging from the following aspects: the influence of NAD+ supplementations on the aging heart; the relationship and cross-talks between NAD+ signaling and other cardiac aging-related signaling pathways; Importantly, the therapeutic potential of targeting NAD+ in delaying heart aging will be discussed. In brief, NAD+ plays a vital role in delaying heart aging. However, the abnormalities such as altered glucose and lipid metabolism, oxidative stress, and calcium overload could also interfere with NAD+ function in the heart. Therefore, the specific physiopathology of the aging heart should be considered before applying NAD+ supplementations. We believe that this article will help augment our understanding of heart aging mechanisms. In the meantime, it provides invaluable insights into possible therapeutic strategies for preventing age-related heart diseases in clinical settings.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
NAD+ Deficiency Is a Common Central Pathological Factor of a Number of Diseases and Aging: Mechanisms and Therapeutic Implications.Antioxid Redox Signal. 2019 Feb 20;30(6):890-905. doi: 10.1089/ars.2017.7445. Epub 2018 Feb 7. Antioxid Redox Signal. 2019. PMID: 29295624 Review.
-
Implications of NAD+ Metabolism in the Aging Retina and Retinal Degeneration.Oxid Med Cell Longev. 2020 May 9;2020:2692794. doi: 10.1155/2020/2692794. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32454935 Free PMC article. Review.
-
Kynurenine pathway, NAD+ synthesis, and mitochondrial function: Targeting tryptophan metabolism to promote longevity and healthspan.Exp Gerontol. 2020 Apr;132:110841. doi: 10.1016/j.exger.2020.110841. Epub 2020 Jan 16. Exp Gerontol. 2020. PMID: 31954874 Free PMC article. Review.
-
NAD and the aging process: Role in life, death and everything in between.Mol Cell Endocrinol. 2017 Nov 5;455:62-74. doi: 10.1016/j.mce.2016.11.003. Epub 2016 Nov 5. Mol Cell Endocrinol. 2017. PMID: 27825999 Free PMC article. Review.
-
Exogenous supplemental NAD+ protect myocardium against myocardial ischemic/reperfusion injury in swine model.Am J Transl Res. 2019 Sep 15;11(9):6066-6074. eCollection 2019. Am J Transl Res. 2019. PMID: 31632574 Free PMC article.
Cited by
-
Systemic aging fuels heart failure: Molecular mechanisms and therapeutic avenues.ESC Heart Fail. 2025 Apr;12(2):1059-1080. doi: 10.1002/ehf2.14947. Epub 2024 Jul 22. ESC Heart Fail. 2025. PMID: 39034866 Free PMC article. Review.
-
Loss of cardiac mitochondrial complex I persulfidation impairs NAD+ homeostasis in aging.Redox Biol. 2024 Feb;69:103014. doi: 10.1016/j.redox.2023.103014. Epub 2023 Dec 25. Redox Biol. 2024. PMID: 38171255 Free PMC article.
-
Pleozymes: Pleiotropic Oxidized Carbon Nanozymes Enhance Cellular Metabolic Flexibility.Nanomaterials (Basel). 2024 Dec 15;14(24):2017. doi: 10.3390/nano14242017. Nanomaterials (Basel). 2024. PMID: 39728553 Free PMC article.
-
Nicotinamide Mononucleotide (NMN) Improves the Senescence of Mouse Vascular Smooth Muscle Cells Induced by Ang II Through Activating p-AMPK/KLF4 Pathway.Pharmaceuticals (Basel). 2025 Apr 9;18(4):553. doi: 10.3390/ph18040553. Pharmaceuticals (Basel). 2025. PMID: 40283988 Free PMC article.
-
Mechanisms of endothelial senescence and vascular aging.Biogerontology. 2025 Jun 25;26(4):128. doi: 10.1007/s10522-025-10279-y. Biogerontology. 2025. PMID: 40560245 Review.
References
-
- Partridge L, Fuentealba M, Kennedy BK. The quest to slow ageing through drug discovery. Nat Rev Drug Discov. 2020;19:513–32. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

