Clinical and transcriptomic features of persistent exacerbation-prone severe asthma in U-BIOPRED cohort
- PMID: 35474304
- PMCID: PMC9043117
- DOI: 10.1002/ctm2.816
Clinical and transcriptomic features of persistent exacerbation-prone severe asthma in U-BIOPRED cohort
Abstract
Background: Exacerbation-prone asthma is a feature of severe disease. However, the basis for its persistency remains unclear.
Objectives: To determine the clinical and transcriptomic features of frequent exacerbators (FEs) and persistent FEs (PFEs) in the U-BIOPRED cohort.
Methods: We compared features of FE (≥2 exacerbations in past year) to infrequent exacerbators (IE, <2 exacerbations) and of PFE with repeat ≥2 exacerbations during the following year to persistent IE (PIE). Transcriptomic data in blood, bronchial and nasal epithelial brushings, bronchial biopsies and sputum cells were analysed by gene set variation analysis for 103 gene signatures.
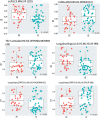
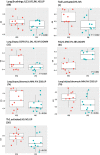
Results: Of 317 patients, 62.4% had FE, of whom 63.6% had PFE, while 37.6% had IE, of whom 61.3% had PIE. Using multivariate analysis, FE was associated with short-acting beta-agonist use, sinusitis and daily oral corticosteroid use, while PFE was associated with eczema, short-acting beta-agonist use and asthma control index. CEA cell adhesion molecule 5 (CEACAM5) was the only differentially expressed transcript in bronchial biopsies between PE and IE. There were no differentially expressed genes in the other four compartments. There were higher expression scores for type 2, T-helper type-17 and type 1 pathway signatures together with those associated with viral infections in bronchial biopsies from FE compared to IE, while there were higher expression scores of type 2, type 1 and steroid insensitivity pathway signatures in bronchial biopsies of PFE compared to PIE.
Conclusion: The FE group and its PFE subgroup are associated with poor asthma control while expressing higher type 1 and type 2 activation pathways compared to IE and PIE, respectively.
Keywords: CEACAM5; asthma exacerbations; frequent exacerbators; persistent frequent exacerbators; severe asthma.
© 2022 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
Dr Singer reports honoraria for lectures and presentations from Novartis Pharma Switzerland and Vertex Pharmaceuticals Switzerland, outside the submitted work. Dr Shaw has received speaker fees from Chiesi and Astra Zeneca and advisory board fees from Adherium, Nuvoair, Astra Zeneca and Chiesi. Dr Loza is employed by and own stock in Johnson & Johnson. Dr. SE Dahlén reports personal fees from AstraZeneca, Cayman Chemicals, GSK, Novartis, Regeneron, Sanofi and Teva for consultancies outside the submitted work. Drs Auffray, Demulder and Lefaudeux report grants from the IMI (U‐BIOPRED n°115010 and eTRIKS n°115446). Dr Fowler has received grants from Boehringer Ingelheim and fees from Chiesi, outside of the current work. Dr Djukanovic reports receiving fees for lectures at symposia organised by Novartis, AstraZeneca and TEVA, consultation for TEVA and Novartis as members of advisory boards, and participation in a scientific discussion about asthma organised by GlaxoSmithKline. He is a co‐founder and current consultant and has shares in Synairgen, a University of Southampton spin out company. Dr Sterk reports grants from public–private funding by the Innovative Medicines Initiative (IMI), outside the submitted work. Dr Chung has received honoraria for participating in Advisory Board meetings of GSK, AZ, Roche, Novartis, Merck, BI, TEVA and Shionogi regarding treatments for asthma, chronic obstructive pulmonary disease and chronic cough and has also been remunerated for speaking engagements. The other authors have no disclosures in relation to this work. U‐BIOPRED was supported by an Innovative Medicines Initiative Joint Undertaking (No. 115010), resources from the European Union's Seventh Framework Programme (FP7/2007‐2013) and EFPIA companies’ in‐kind contribution (
Figures
Comment in
-
Frequent exacerbators in severe asthma: Focus on clinical and transcriptional factors.Clin Transl Med. 2022 May;12(5):e860. doi: 10.1002/ctm2.860. Clin Transl Med. 2022. PMID: 35538891 Free PMC article. No abstract available.
-
From spirometry to spatial omics in pursuit of asthma endotypes.Clin Transl Med. 2022 Sep;12(9):e878. doi: 10.1002/ctm2.878. Clin Transl Med. 2022. PMID: 36149782 Free PMC article. No abstract available.
References
-
- Reddel HK, Taylor DR, Bateman ED, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180(1):59–99. - PubMed
-
- O'Byrne PM, Pedersen S, Lamm CJ, Tan WC, Busse WW. Severe exacerbations and decline in lung function in asthma. Am J Respir Crit Care Med. 2009;179(1):19–24. - PubMed
-
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
