Antibiotic Use and Vaccine Antibody Levels
- PMID: 35474546
- PMCID: PMC9648114
- DOI: 10.1542/peds.2021-052061
Antibiotic Use and Vaccine Antibody Levels
Abstract
Background: The majority of children are prescribed antibiotics in the first 2 years of life while vaccine-induced immunity develops. Researchers have suggested a negative association of antibiotic use with vaccine-induced immunity in adults, but data are lacking in children.
Methods: From 2006 to 2016, children aged 6 to 24 months were observed in a cohort study. A retrospective, unplanned secondary analysis of the medical record regarding antibiotic prescriptions and vaccine antibody measurements was undertaken concurrently. Antibody measurements relative to diphtheria-tetanus-acellular pertussis (DTaP), inactivated polio (IPV), Haemophilus influenzae type b (Hib), and pneumococcal conjugate (PCV) vaccines were made.
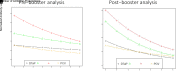
Results: In total, 560 children were compared (342 with and 218 without antibiotic prescriptions). Vaccine-induced antibody levels to several DTaP and PCV antigens were lower (P < .05) in children given antibiotics. A higher frequency of vaccine-induced antibodies below protective levels in children given antibiotics occurred at 9 and 12 months of age (P < .05). Antibiotic courses over time was negatively associated with vaccine-induced antibody levels. For each antibiotic course the child received, prebooster antibody levels to DTaP antigens were reduced by 5.8%, Hib by 6.8%, IPV by 11.3%, and PCV by 10.4% (all P ≤ .05), and postbooster antibody levels to DTaP antigens were reduced by 18.1%, Hib by 21.3%, IPV by 18.9%, and PCV by 12.2% (all P < .05).
Conclusions: Antibiotic use in children <2 years of age is associated with lower vaccine-induced antibody levels to several vaccines.
Copyright © 2022 by the American Academy of Pediatrics.
Conflict of interest statement
Figures

Comment in
-
Antibiotics and Immunizations: A Complex Interaction.Pediatrics. 2022 May 1;149(5):e2021055610. doi: 10.1542/peds.2021-055610. Pediatrics. 2022. PMID: 35474544 No abstract available.
References
-
- Wang H, Dwyer-Lindgren L, Lofgren KT, et al. . Age-specific and sex-specific mortality in 187 countries, 1970-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2071–2094 - PubMed
-
- Payton T, Girgenti D, Frenck RW, et al. . Immunogenicity, safety, and tolerability of 3 lots of 13-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in the United States. Pediatr Infect Dis J. 2013; 32(8):871–880 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

