Spontaneous Coassembly of the Protein Terthiophene into Fluorescent Electroactive Microfibers in 2D and 3D Cell Cultures
- PMID: 35474798
- PMCID: PMC9026133
- DOI: 10.1021/acsomega.1c06677
Spontaneous Coassembly of the Protein Terthiophene into Fluorescent Electroactive Microfibers in 2D and 3D Cell Cultures
Abstract
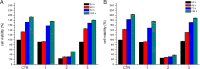
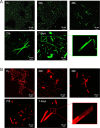
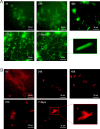
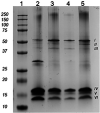
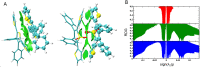
Protein-based microfibers are biomaterials of paramount importance in materials science, nanotechnology, and medicine. Here we describe the spontaneous in situ formation and secretion of nanostructured protein microfibers in 2D and 3D cell cultures of 3T3 fibroblasts and B104 neuroblastoma cells upon treatment with a micromolar solution of either unmodified terthiophene or terthiophene modified by mono-oxygenation (thiophene → thiophene S-oxide) or dioxygenation (thiophene → thiophene S,S-dioxide) of the inner ring. We demonstrate via metabolic cytotoxicity tests that modification to the S-oxide leads to a severe drop in cell viability. By contrast, unmodified terthiophene and the respective S,S-dioxide cause no harm to the cells and lead to the formation and secretion of fluorescent and electroactive protein-fluorophore coassembled microfibers with a large aspect ratio, a micrometer-sized length and width, and a nanometer-sized thickness, as monitored in real-time by laser scanning confocal microscopy (LSCM). With respect to the microfibers formed by unmodified terthiophene, those formed by the S,S-dioxide display markedly red-shifted fluorescence and an increased n-type character of the material, as shown by macroscopic Kelvin probe in agreement with cyclovoltammetry data. Electrophoretic analyses and Q-TOF mass spectrometry of the isolated microfibers indicate that in all cases the prevalent proteins present are vimentin and histone H4, thus revealing the capability of these fluorophores to selectively coassemble with these proteins. Finally, DFT calculations help to illuminate the fluorophore-fluorophore intermolecular interactions contributing to the formation of the microfibers.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
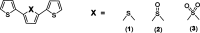






References
-
- Wang M.-D.; Huang Y.-Q.; Wang H.. In Vivo Self-Assembly of Polypeptide-Based Nanomaterials. In Handbook of Macrocyclic Supramolecular Assembly; Liu Y., Chen Y., Zhang H.-Y., Eds.; Springer Singapore, 2019; pp 1023–1043.
-
- Schreiber S. L. Chemical genetics resulting from a passion for synthetic organic chemistry. Bioorg. Med. Chem. 1998, 6 (8), 1127–1152. 10.1016/S0968-0896(98)00126-6. - DOI - PubMed
-
From NLM.
- Schreiber S. L. A Chemical Biology View of Bioactive Small Molecules and a Binder-Based Approach to Connect Biology to Precision Medicines. Isr. J. Chem. 2019, 59 (1–2), 52–59. 10.1002/ijch.201800113. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

