First-Principles Study of Three-Dimensional Electrides Containing One-Dimensional [Ba3N]3+ Chains
- PMID: 35474803
- PMCID: PMC9026116
- DOI: 10.1021/acsomega.2c00956
First-Principles Study of Three-Dimensional Electrides Containing One-Dimensional [Ba3N]3+ Chains
Abstract
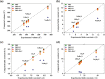
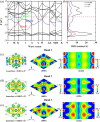
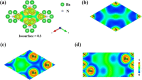
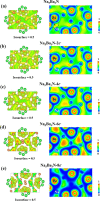
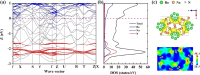
Electrides, a unique type of compound where electrons act as anions, have a high electron mobility and a low work function, which makes them promising for applications in electronic devices and high-performance catalysts. The discovery of novel electrides and the expansion of the electride family have great significance for their promising applications. Herein, we reported four three-dimensional (3D) electrides by coupling crystal structure database searches and first-principles electronic structure analysis. Subnitrides (Ba3N, LiBa3N, NaBa3N, and Na5Ba3N) containing one-dimensional (1D) [Ba3N]3+ chains are identified as 3D electrides for the first time. The anionic electrons are confined in the 3D interstitial space of Ba3N, LiBa3N, NaBa3N, and Na5Ba3N. Interestingly, with the increase of Na content, the excess electrons of Na5Ba3N play two roles of metallic bonding and anionic electrons. Therefore, the subnitrides containing 1D [Ba3N]3+ chains can be regarded as a new family of 3D electrides, where anionic electrons reside in the 3D interstitial spaces and provide a conduction path. These materials not only are experimentally synthesizable 3D electrides but also are promising to be exfoliated into advanced 1D nanowire materials. Furthermore, our work suggests a discovery strategy of novel electrides based on one parent framework like [Ba3N]3+ chains, which would accelerate the mining of electrides from the crystal structure database.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
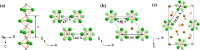



Similar articles
-
BaCu, a Two-Dimensional Electride with Cu Anions.J Am Chem Soc. 2024 Jun 26;146(25):17508-17516. doi: 10.1021/jacs.4c05723. Epub 2024 Jun 11. J Am Chem Soc. 2024. PMID: 38861394
-
Advances in Materials and Applications of Inorganic Electrides.Chem Rev. 2021 Mar 10;121(5):3121-3185. doi: 10.1021/acs.chemrev.0c01071. Epub 2021 Feb 19. Chem Rev. 2021. PMID: 33606511
-
Electrides: early examples of quantum confinement.Acc Chem Res. 2009 Oct 20;42(10):1564-72. doi: 10.1021/ar9000857. Acc Chem Res. 2009. PMID: 19645438
-
First-Principles Prediction of Thermodynamically Stable Two-Dimensional Electrides.J Am Chem Soc. 2016 Nov 30;138(47):15336-15344. doi: 10.1021/jacs.6b05586. Epub 2016 Nov 16. J Am Chem Soc. 2016. PMID: 27764942
-
Molecular Electrides: An In Silico Perspective.Chemphyschem. 2022 Dec 5;23(23):e202200329. doi: 10.1002/cphc.202200329. Epub 2022 Sep 1. Chemphyschem. 2022. PMID: 35894262 Review.
References
-
- Liu C.; Nikolaev S. A.; Ren W.; Burton L. A. Electrides: a review. J. Mater. Chem. C 2020, 8, 10551–10567. 10.1039/D0TC01165G. - DOI
-
- Tada T.; Wang J.; Hosono H. First principles evolutionary search for new electrides along the dimensionality of anionic electrons. J. Comput. Chem., Jpn. 2017, 16, 135–138. 10.2477/jccj.2017-0067. - DOI
-
- Zhu Q.; Frolov T.; Choudhary K. Computational discovery of inorganic electrides from an automated screening. Matter 2019, 1, 1293–1303. 10.1016/j.matt.2019.06.017. - DOI
LinkOut - more resources
Full Text Sources

