Microbial Carbonation of Monocalcium Silicate
- PMID: 35474837
- PMCID: PMC9025989
- DOI: 10.1021/acsomega.1c05264
Microbial Carbonation of Monocalcium Silicate
Abstract
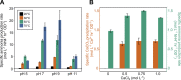
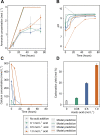
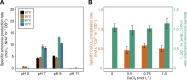
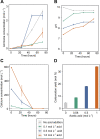
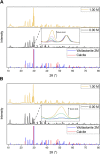
Biocement formed through microbially induced calcium carbonate precipitation (MICP) is an emerging biotechnology focused on reducing the environmental impact of concrete production. In this system, CO2 species are provided via ureolysis by Sporosarcina pasteurii (S. pasteurii) to carbonate monocalcium silicate for MICP. This is one of the first studies of its kind that uses a solid-state calcium source, while prior work has used highly soluble forms. Our study focuses on microbial physiological, chemical thermodynamic, and kinetic studies of MICP. Monocalcium silicate incongruently dissolves to form soluble calcium, which must be coupled with CO2 release to form calcium carbonate. Chemical kinetic modeling shows that calcium solubility is the rate-limiting step, but the addition of organic acids significantly increases the solubility, enabling extensive carbonation to proceed up to 37 mol %. The microbial urease activity by S. pasteurii is active up to pH 11, 70 °C, and 1 mol L-1 CaCl2, producing calcite as a means of solidification. Cell-free extracts are also effective albeit less robust at extreme pH, producing calcite with different physical properties. Together, these data help determine the chemical, biological, and thermodynamic parameters critical for scaling microbial carbonation of monocalcium silicate to high-density cement and concrete.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Worrell E.; Price L.; Martin N.; Hendriks C.; Meida L. O. Carbon dioxide emissions from the global cement industry. Annu. Rev. Energy 2001, 26, 303–329.
-
- Tracking Industrial Energy Efficiency and CO2 Emissions. IEA, June 2007. https://www.iea.org/reports/tracking-industrial-energy-efficiency-and-co... (accessed 2021-09-22).
-
- Huang H.; Wang T.; Kolosz B.; Andresen J.; Garcia S.; Fang M.; Maroto-Valer M. M. Life-cycle assessment of emerging CO2 mineral carbonation-cured concrete blocks: Comparative analysis of CO2 reduction potential and optimization of environmental impacts. J. Cleaner Prod. 2019, 241, 11835910.1016/j.jclepro.2019.118359. - DOI
-
- Riman R. E.; Gupta S.; Atakan V.; Li Q.. Bonding element, bonding matrix and composite material having the bonding element, and method of manufacturing thereof. US 9,868,667, 2018.
-
- Riman R. E.; Atakan V.; Kuppler J. P.; Smith K. M.. Precursors and transport methods for hydrothermal liquid phase sintering (HLPS). US 9,266,147, 2016.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

