Allogeneic haematopoietic stem cell transplantation resets T- and B-cell compartments in sickle cell disease patients
- PMID: 35474905
- PMCID: PMC9035210
- DOI: 10.1002/cti2.1389
Allogeneic haematopoietic stem cell transplantation resets T- and B-cell compartments in sickle cell disease patients
Abstract
Objectives: Allogeneic haematopoietic stem cell transplantation (allo-HSCT) is the only currently available curative treatment for sickle cell disease (SCD). Here, we comprehensively evaluated the reconstitution of T- and B-cell compartments in 29 SCD patients treated with allo-HSCT and how it correlated with the development of acute graft-versus-host disease (aGvHD).
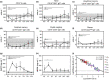
Methods: T-cell neogenesis was assessed by quantification of signal-joint and β-chain TCR excision circles. B-cell neogenesis was evaluated by quantification of signal-joint and coding-joint K-chain recombination excision circles. T- and B-cell peripheral subset numbers were assessed by flow cytometry.
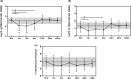
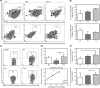
Results: Before allo-HSCT (baseline), T-cell neogenesis was normal in SCD patients compared with age-, gender- and ethnicity-matched healthy controls. Following allo-HSCT, T-cell neogenesis declined but was fully restored to healthy control levels at one year post-transplantation. Peripheral T-cell subset counts were fully restored only at 24 months post-transplantation. Occurrence of acute graft-versus-host disease (aGvHD) transiently affected T- and B-cell neogenesis and overall reconstitution of T- and B-cell peripheral subsets. B-cell neogenesis was significantly higher in SCD patients at baseline than in healthy controls, remaining high throughout the follow-up after allo-HSCT. Notably, after transplantation SCD patients showed increased frequencies of IL-10-producing B-regulatory cells and IgM+ memory B-cell subsets compared with baseline levels and with healthy controls.
Conclusion: Our findings revealed that the T- and B-cell compartments were normally reconstituted in SCD patients after allo-HSCT. In addition, the increase of IL-10-producing B-regulatory cells may contribute to improve immune regulation and homeostasis after transplantation.
Keywords: B‐cell neogenesis; T‐cell neogenesis; allogeneic haematopoietic stem cell transplantation; peripheral homeostasis; sickle cell disease.
© 2022 The Authors. Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
