Immunotherapeutic nanoparticles: From autoimmune disease control to the development of vaccines
- PMID: 35475005
- PMCID: PMC9023085
- DOI: 10.1016/j.bioadv.2022.212726
Immunotherapeutic nanoparticles: From autoimmune disease control to the development of vaccines
Abstract
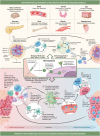
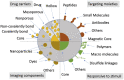
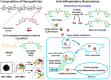
The development of nanoparticles (NPs) with potential therapeutic uses represents an area of vast interest in the scientific community during the last years. Recently, the pandemic caused by COVID-19 motivated a race for vaccines creation to overcome the crisis generated. This is a good demonstration that nanotechnology will most likely be the basis of future immunotherapy. Moreover, the number of publications based on nanosystems has significantly increased in recent years and it is expected that most of these developments can go on to experimentation in clinical stages soon. The therapeutic use of NPs to combat different diseases such as cancer, allergies or autoimmune diseases will depend on their characteristics, their targets, and the transported molecules. This review presents an in-depth analysis of recent advances that have been developed in order to obtain novel nanoparticulate based tools for the treatment of allergies, autoimmune diseases and for their use in vaccines. Moreover, it is highlighted that by providing targeted delivery an increase in the potential of vaccines to induce an immune response is expected in the future. Definitively, the here gathered analysis is a good demonstration that nanotechnology will be the basis of future immunotherapy.
Keywords: Allergy; Autoimmune disease; Immune stimulation; Immunomodulation therapy; Immunosuppressants; Nanoparticles.
© 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources

