Amelioration of ligamentum flavum hypertrophy using umbilical cord mesenchymal stromal cell-derived extracellular vesicles
- PMID: 35475028
- PMCID: PMC9014323
- DOI: 10.1016/j.bioactmat.2022.03.042
Amelioration of ligamentum flavum hypertrophy using umbilical cord mesenchymal stromal cell-derived extracellular vesicles
Abstract
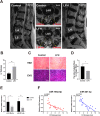
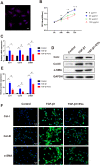
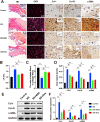
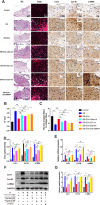
Ligamentum flavum (LF) hypertrophy (LFH) has been recognised as one of the key contributors to lumbar spinal stenosis. Currently, no effective methods are available to ameliorate this hypertrophy. In this study, human umbilical cord mesenchymal stromal cell-derived extracellular vesicles (hUCMSC-EVs) were introduced for the first time as promising vehicles for drug delivery to treat LFH. The downregulation of miR-146a-5p and miR-221-3p expressions in human LF tissues negatively correlated with increased LF thickness. The hUCMSC-EVs enriched with these two miRNAs significantly suppressed LFH in vivo and notably ameliorated the progression of transforming growth factor β1(TGF-β1)-induced fibrosis in vitro after delivering these two miRNAs to mouse LF cells. The results further demonstrated that miR-146a-5p and miR-221-3p directly bonded to the 3'-UTR regions of SMAD4 mRNA, thereby inhibiting the TGF-β/SMAD4 signalling pathway. Therefore, this translational study determined the effectiveness of a hUCMSC-EVs-based approach for the treatment of LFH and revealed the critical target of miR-146a-5p and miR-221-3p. Our findings provide new insights into promising therapeutics using a hUCMSC-EVs-based delivery system for patients with lumbar spinal stenosis.
Keywords: ECM, extracellular matrix; Extracellular vesicle; Fibrosis; LF, Ligamentum flavum; LFH, Ligamentum flavum hypertrophy; LSS, Lumbar spinal stenosis; Ligamentum flavum hypertrophy; MRI, magnetic resonance imaging; SMAD, mothers against the decapentaplegic homolog; TGF-β1, transforming growth factor-β1; Umbilical cord mesenchymal stromal cells; hUCMSC-EVs, human umbilical cord mesenchymal stromal cell-derived extracellular vesicles; miR-146a-5p; miR-221-3p.
© 2022 The Authors.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

