A nonchlorinated solvent-processed polymer semiconductor for high-performance ambipolar transistors
- PMID: 35475218
- PMCID: PMC9031015
- DOI: 10.1093/nsr/nwab145
A nonchlorinated solvent-processed polymer semiconductor for high-performance ambipolar transistors
Abstract
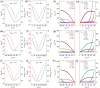
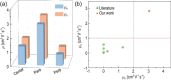
Ambipolar polymer semiconductors are potentially serviceable for logic circuits, light-emitting field-effect transistors (LFETs) and polymer solar cells (PSCs). Although several high-performance ambipolar polymers have been developed, their optoelectronic devices are generally processed from toxic chlorinated solvents. To achieve the commercial applications of organic FETs (OFETs), the polymers should be processed from nonchlorinated solvents, instead of chlorinated solvents. However, most conjugated polymers show poor solubility in nonchlorinated solvents. It is of great importance to develop ambipolar polymers that can be processed from nonchlorinated solvents. Here, we develop a nonchlorinated solvent processed polymer named poly[7-fluoro-N, N'-di(4-decyltetradecyl)-7'-azaisoindigo-6',6″-(thieno[3,2-b]thiophene-2,5-diyl)-7‴-fluoro-N″, N‴-di(4-decyltetradecyl)-7″-azaisoindigo-6,6‴-([2,2″-bithiophene]-5,5″-diyl)] (PITTI-BT) by designing a monomer with a large molar mass. The polymer displays good solubility in p-xylene (PX). Well-aligned films of PITTI-BT are achieved by an off-center spin-coating (SC) method. Based on the high-quality films, the OFETs fabricated from PX solution achieve record ambipolar performance with hole and electron mobilities of 3.06 and 2.81 cm2 V-1 s-1, respectively. The combination of nonchlorinated solvents and good alignment process offers an effective and eco-friendly approach to obtain high-performance ambipolar transistors.
Keywords: alignment; ambipolar transistor; high performance; nonchlorinated solvent.
© The Author(s) 2021. Published by Oxford University Press on behalf of China Science Publishing & Media Ltd.
Figures




References
-
- Yang J, Zhao Z, Wang Set al. . Insight into high-performance conjugated polymers for organic field-effect transistors. Chem 2018; 4: 2748–85.10.1016/j.chempr.2018.08.005 - DOI
-
- Zhao J, Li Y, Yang Get al. . Efficient organic solar cells processed from hydrocarbon solvents. Nat Energy 2016; 1: 15027.10.1038/nenergy.2015.27 - DOI
LinkOut - more resources
Full Text Sources
