Tocilizumab in patients hospitalised with COVID-19 pneumonia: Efficacy, safety, viral clearance, and antibody response from a randomised controlled trial (COVACTA)
- PMID: 35475258
- PMCID: PMC9022847
- DOI: 10.1016/j.eclinm.2022.101409
Tocilizumab in patients hospitalised with COVID-19 pneumonia: Efficacy, safety, viral clearance, and antibody response from a randomised controlled trial (COVACTA)
Abstract
Background: In COVACTA, a randomised, placebo-controlled trial in patients hospitalised with coronavirus disease-19 (COVID-19), tocilizumab did not improve 28-day mortality, but shortened hospital and intensive care unit stay. Longer-term effects of tocilizumab in patients with COVID-19 are unknown. Therefore, the efficacy and safety of tocilizumab in COVID-19 beyond day 28 and its impact on Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) clearance and antibody response in COVACTA were investigated.
Methods: Adults in Europe and North America hospitalised with COVID-19 (N = 452) between April 3, 2020 and May 28, 2020 were randomly assigned (2:1) to double-blind intravenous tocilizumab or placebo and assessed for efficacy and safety through day 60. Assessments included mortality, time to hospital discharge, SARS-CoV-2 viral load in nasopharyngeal swab and serum samples, and neutralising anti-SARS-CoV-2 antibodies in serum. ClinicalTrials.gov registration: NCT04320615.
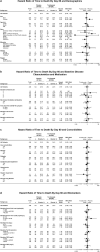
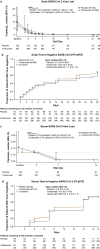
Findings: By day 60, 24·5% (72/294) of patients in the tocilizumab arm and 25·0% (36/144) in the placebo arm died (weighted difference -0·5% [95% CI -9·1 to 8·0]), and 67·0% (197/294) in the tocilizumab arm and 63·9% (92/144) in the placebo arm were discharged from the hospital. Serious infections occurred in 24·1% (71/295) of patients in the tocilizumab arm and 29·4% (42/143) in the placebo arm. Median time to negative reverse transcriptase-quantitative polymerase chain reaction result in nasopharyngeal/oropharyngeal samples was 15·0 days (95% CI 14·0 to 21·0) in the tocilizumab arm and 21·0 days (95% CI 14·0 to 28·0) in the placebo arm. All tested patients had positive test results for neutralising anti-SARS-CoV-2 antibodies at day 60.
Interpretation: There was no mortality benefit with tocilizumab through day 60. Tocilizumab did not impair viral clearance or host immune response, and no new safety signals were observed. Future investigations may explore potential biomarkers to optimize patient selection for tocilizumab treatment and combination therapy with other treatments.
Funding: F. Hoffmann-La Roche Ltd and the US Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, under OT number HHSO100201800036C.
Keywords: Coronavirus disease 2019; Interleukin-6; Randomised controlled trial; Severe acute respiratory syndrome coronavirus-2; Tocilizumab; Viral load.
© 2022 The Author(s).
Conflict of interest statement
Ivan O. Rosas: Grant from Roche/Genentech during the conduct of the study; grant and personal fees from Genentech outside the submitted work; and personal fees from Boehringer and Bristol Myers Squibb outside the submitted work Norbert Bräu: Grant support to institution from Roche/Genentech during the conduct of the study; grants from Gilead Sciences outside the submitted work Ronaldo C. Go: Consulting fees from F. Hoffmann-La Roche outside the submitted work Atul Malhotra: Grants from the National Institutes of Health; personal fees from LivaNova, Corvus, and Equillium; institutional funding from RedMed outside the submitted work Bradley D. Hunter: Personal fees from Kite Pharmaceuticals and Novartis outside the submitted work Sanjay Bhagani: Grants and personal fees from Gilead Sciences, Roche, and ViiV outside the submitted work Sinisa Savic: Grants and personal fees from Novartis and SOBI outside the submitted work Ivor S. Douglas: Grant support to institution from Roche/Genentech during the conduct of the study Andrew Ustianowski: Grant support to institution from Roche/Genentech during the conduct of the study Jordi Carratalà: Grant from Roche/Genentech during the conduct of the study; grant and personal fees from Gilead Sciences outside the submitted work Thomas Benfield: Grants from Novo Nordisk Foundation, Simonsen Foundation, GlaxoSmithKline, Pfizer, Gilead, Lundbeck Foundation, Kai Hansen Foundation and Erik and Susanna Olesen's charitable fund; personal fees from GlaxoSmithKline, Pfizer, Boehringer Ingelheim, Gilead, MSD and PentaBase A/S outside the submitted work Paolo Bonfanti: Personal fees from ViiV, Gilead, Janssen, and Merck outside the submitted work. Cor H. van der Leest: Personal fees related to the submitted work; personal fees from Bristol Myers Squib, Merck Sharp & Dohme, AbbVie, Boehringer Ingelheim, Roche, and AstraZeneca outside the submitted work Charles Edouard Luyt: Grant support to institution from Roche/Genentech during the conduct of the study; personal fees from Carmat, Merck, bioMérieux, Thermo Fischer Brahms, Bayer Healthcare, and Faron outside the submitted work Rebecca N. Bauer: Employee of Genentech and holder of stock/stock options in Roche Fang Cai: Former employee of Genentech; patent pending to Genentech for biomarkers for predicting response to an IL-6 antagonist (P36367-US) Ivan T. Lee: Former clinical research fellow of Genentech/Roche; received funding from Genentech/Roche during the conduct of the study Balpreet Matharu: Employee of Roche Products Ltd Louis Metcalf: Employee of and owns shares in Roche Steffen Wildum: Employee of F Hoffmann-La Roche Emily Graham: Employee of Roche Products Ltd Larry Tsai: Employee of Genentech/Roche; unpublished patent pending for ‘Method for treating pneumonia, including COVID-19 pneumonia, with an IL-6 antagonist’ Min Bao: Grant from Biomedical Advanced Research and Development Authority (BARDA) for the COVACTA study; employee of Genentech/Roche; unpublished patent pending for ‘Method for treating pneumonia, including COVID-19 pneumonia, with an IL-6 antagonist’ All other authors have nothing to disclose.
Figures
References
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

