Eptacog beta efficacy and safety in the treatment and control of bleeding in paediatric subjects (<12 years) with haemophilia A or B with inhibitors
- PMID: 35475308
- PMCID: PMC9542908
- DOI: 10.1111/hae.14563
Eptacog beta efficacy and safety in the treatment and control of bleeding in paediatric subjects (<12 years) with haemophilia A or B with inhibitors
Abstract
Introduction: Eptacog beta is a new recombinant activated human factor VII bypassing agent approved in the United States for the treatment and control of bleeding in patients with haemophilia A or B with inhibitors 12 years of age or older.
Aim: To prospectively assess in a phase 3 clinical trial (PERSEPT 2) eptacog beta efficacy and safety for treatment of bleeding in children <12 years of age with haemophilia A or B with inhibitors.
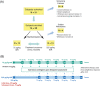
Methods: Using a randomised crossover design, subjects received initial doses of 75 or 225 μg/kg eptacog beta followed by 75 μg/kg dosing at predefined intervals (as determined by clinical response) to treat bleeding episodes (BEs). Treatment success criteria included a haemostasis evaluation of 'excellent' or 'good' without use of additional eptacog beta, alternative haemostatic agent or blood product, and no increase in pain following the first 'excellent' or 'good' assessment.
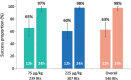
Results: Treatment success proportions in 25 subjects (1-11 years) who experienced 546 mild or moderate BEs were 65% in the 75 μg/kg initial dose regimen (IDR) and 60% in the 225 μg/kg IDR 12 h following initial eptacog beta infusion. By 24 h, the treatment success proportions were 97% for the 75 μg/kg IDR and 98% for the 225 μg/kg IDR. No thrombotic events, allergic reactions, neutralising antibodies or treatment-related adverse events were reported.
Conclusion: Both 75 and 225 μg/kg eptacog beta IDRs provided safe and effective treatment and control of bleeding in children <12 years of age.
Keywords: PERSEPT; eptacog beta; haemophilia; inhibitors; paediatric; recombinant FVIIa.
© 2022 The Authors. Haemophilia published by John Wiley & Sons Ltd.
Figures
References
-
- Scalone L, Mantovani LG, Mannucci PM, Gringeri A, COCIS Study Investigators . Quality of life is associated to the orthopaedic status in haemophilic patients with inhibitors. Haemophilia. 2006;12(2):154‐162. - PubMed
-
- Neufeld EJ, Recht M, Sabio H, et al. Effect of acute bleeding on daily quality of life assessments in patients with congenital hemophilia with inhibitors and their families: observations from the dosing observational study in hemophilia. Value Health. 2012;15(6):916‐925. - PubMed
-
- Walsh CE, Jimenez‐Yuste V, Auerswald G, Grancha S. The burden of inhibitors in haemophilia patients. Thromb Haemost. 2016;116 (Suppl. 1):S10‐17. - PubMed
-
- Astermark J. Overview of inhibitors. Semin Hematol. 2006;43(Suppl 4):S3‐7. - PubMed

