No Effect of Lactobacillus rhamnosus GG on Eradication of Colonization by Vancomycin-Resistant Enterococcus faecium or Microbiome Diversity in Hospitalized Adult Patients
- PMID: 35475684
- PMCID: PMC9241610
- DOI: 10.1128/spectrum.02348-21
No Effect of Lactobacillus rhamnosus GG on Eradication of Colonization by Vancomycin-Resistant Enterococcus faecium or Microbiome Diversity in Hospitalized Adult Patients
Abstract
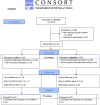
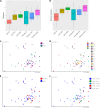
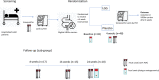
The purpose of this trial was to evaluate the efficacy of a 4-week supplementation of Lactobacillus rhamnosus GG (LGG) in eliminating the gastrointestinal carrier state of vancomycin-resistant Enterococcus faecium (VREfm) in hospitalized adults. The primary outcome of the study was the number of patients with cleared VREfm colonization after the 4-week intervention. Secondary outcomes were clearance of VREfm colonization at weeks 8, 16, and 24, number of VREfm infections (isolated from nonintestinal foci), and changes in fecal microbiome diversity after the intervention. The trial was a multicenter, randomized, double-blind, placebo-controlled trial in hospitalized adult VREfm carriers. Patients were enrolled and randomized to receive 60 billion CFU of LGG daily or placebo for 4 weeks. For a subgroup of patients, rectal swabs for VREfm were collected also at 8, 16, and 24 weeks and analyzed using shotgun metagenomics. Patients ingesting a minimum of 50% of the probiotic during the 4-week intervention were included in subsequent outcome analyses (48 of 81 patients). Twelve of 21 patients in the LGG group (57%) compared to 15 of 27 patients in the placebo group (56%) cleared their VREfm carriage. Eighteen patients completed the entire 24-week intervention with the same minimum compliancy. Of these, almost 90% in both groups cleared their VREfm carriage. We found a statistically significant difference between VREfm clearers and nonclearers regarding metronidazole and vancomycin usage as well as length of hospitalization after inclusion. The microbiome analyses revealed no significant difference in alpha diversity between the LGG and the placebo group. Beta diversity differed between the groups and the different time points. This study did not show an effect of LGG in eradication of VREfm after a 4-week intervention. IMPORTANCE Whereas other studies exploring the effect of L. rhamnosus in clearing VREfm from the intestine included children and adults, with a wider age range, our study consisted of a geriatric patient cohort. The natural clearance of VREfm in this study was almost 60% after 4 weeks, thus much higher than described previously. Also, this study characterizes the microbiome of VREfm patients in detail. This article showed no effect of the probiotic L. rhamnosus in clearing VREfm from the intestine of patients.
Keywords: Lactobacillus rhamnosus; gut microbiome; probiotics; vancomycin-resistant Enterococcus faecium.
Conflict of interest statement
The authors declare a conflict of interest. The probiotics and placebo have been donated by Chr. Hansen A/S (Hoersholm, Denmark). The study was funded by an unrestricted grant from Chr. Hansen A/S (Hoersholm, Denmark) as well as by the Hvidovre University Hospital research fund. The funding sources do not have a role in data collection, interpretation of analysis, writing of the manuscript or decision to submit publication.
Figures
References
-
- Pinholt M, Ostergaard C, Arpi M, Bruun NE, Schønheyder HC, Gradel KO, Søgaard M, Knudsen JD, Danish Collaborative Bacteraemia Network (DACOBAN). 2014. Incidence, clinical characteristics and 30-day mortality of enterococcal bacteraemia in Denmark 2006–2009: a population-based cohort study. Clin Microbiol Infect 20:145–151. doi:10.1111/1469-0691.12236. - DOI - PubMed
-
- Toft A, Nordestgaard MM, Holm A, Hammerum AM, Hasman H, Danmap JU. 2020. Use of antimicrobial agents and occurrence of antimicrobial resistance in bacteria from food animals, food and humans in Denmark. ISSN 1600–2032. Available from www.danmap.org.
-
- Pinholt M, Bayliss SC, Gumpert H, Worning P, Jensen VVS, Pedersen M, Feil EJ, Westh H. 2019. WGS of 1058 Enterococcus faecium from Copenhagen, Denmark, reveals rapid clonal expansion of vancomycin-resistant clone ST80 combined with widespread dissemination of a vanA-containing plasmid and acquisition of a heterogeneous accessory genome. J Antimicrob Chemother 74:1776–1785. doi:10.1093/jac/dkz118. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

